Page 9 - JCTR-9-4
P. 9
AboEl-Azm et al. | Journal of Clinical and Translational Research 2023; 9(4): 222-235 225
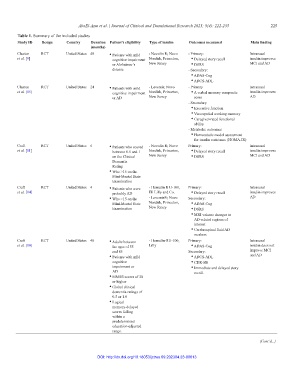
Table 1. Summary of the included studies
Study ID Design Country Duration Patient’s eligibility Type of insulin Outcomes measured Main finding
(months)
Claxton RCT United States 48 • Patients with mild - Novolin R; Novo - Primary: Intranasal
et al. [9] cognitive impairment Nordisk, Princeton, • Delayed story recall insulin improves
or Alzheimer’s New Jersey • DSRS MCI and AD
disease - Secondary:
• ADAS-Cog
• ADCS-ADL
Claxton RCT United States 24 • Patients with mild - Levemir; Novo - Primary Intranasal
et al. [15] cognitive impairment Nordisk, Princeton, • A verbal memory composite insulin improves
or AD New Jersey score AD
- Secondary
• Executive function
• Visuospatial working memory
• Caregiver-rated functional
ability
- Metabolic outcomes
• Homeostatic model assessment
for insulin resistance (HOMA-IR)
Craft RCT United States 6 • Patients who scored - Novolin R; Novo Primary: Intranasal
et al. [11] between 0.5 and 1 Nordisk, Princeton, • Delayed story recall insulin improves
on the Clinical New Jersey • DSRS MCI and AD
Dementia
Rating
• Who >15 on the
Mini-Mental State
Examination
Craft RCT United States 4 • Patients who were - Humulin R U-100, Primary: Intranasal
et al. [14] probably AD Eli Lilly and Co. • Delayed story recall insulin improves
• Who >15 on the - Levemir®; Novo Secondary: AD
Mini-Mental State Nordisk, Princeton, • ADAS-Cog
Examination New Jersey • DSRS
• MRI volume changes in
AD-related regions of
interest
• Cerebrospinal fluid AD
markers
Craft RCT United States 48 • Adults between - Humulin-RU-100; Primary: Intranasal
et al. [10] the ages of 55 Lilly • ADAS-Cog insulin does not
and 85 Secondary: improve MCI
• Patients with mild • ADCS-ADL and AD
cognitive • CDR-SB
impairment or • Immediate and delayed story
AD recall.
• MMSE scores of 20
or higher
• Global clinical
dementia ratings of
0.5 or 1.0
• Logical
memory-delayed
scores falling
within a
predetermined
education-adjusted
range.
(Cont’d...)
DOI: http://dx.doi.org/10.18053/jctres.09.202304.23-00013