Page 28 - MI-2-3
P. 28
Microbes & Immunity Correlation between VZV and cancer
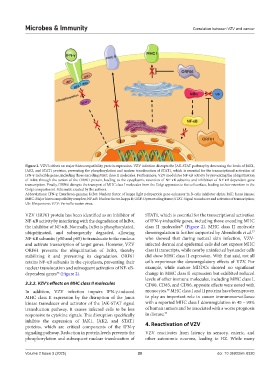
Figure 2. VZV’s effects on major histocompatibility protein expression. VZV infection disrupts the JAK-STAT pathway by decreasing the levels of JAK1,
JAK2, and STAT1 proteins, preventing the phosphorylation and nuclear translocation of STAT1, which is essential for the transcriptional activation of
IFN-γ-inducible genes, including those encoding MHC class II molecules. Furthermore, VZV modulates NF-κB activity by preventing the ubiquitination
of IκBα through the action of the ORF61 protein, leading to the cytoplasmic retention of NF-κB subunits and inhibition of NF-κB-dependent gene
transcription. Finally, ORF66 disrupts the transport of MHC class I molecules from the Golgi apparatus to the cell surface, leading to their retention in the
Golgi compartment. Schematic created by the authors.
Abbreviations: IFN-γ: Interferon-gamma; IκBα: Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; JAK: Janus kinase;
MHC: Major histocompatibility complex; NF-κB: Nuclear factor-kappa B; ORF: Open reading frame; STAT: Signal transducer and activator of transcription;
Ub: Ubiquinone; VZV: Varicella-zoster virus.
VZV ORF61 protein has been identified as an inhibitor of STAT1, which is essential for the transcriptional activation
NF-κB activity by interfering with the degradation of IκBα, of IFN-γ-inducible genes, including those encoding MHC
the inhibitor of NF-κB. Normally, IκBα is phosphorylated, class II molecules (Figure 2). MHC class II molecule
30
77
ubiquitinated, and subsequently degraded, allowing downregulation is further supported by Abendroth et al.
NF-κB subunits (p50 and p65) to translocate to the nucleus who showed that during natural skin infection, VZV-
and activate transcription of target genes. However, VZV infected dermal and epidermal cells did not express MHC
ORF61 prevents the ubiquitination of IκBα, thereby class II transcripts, while nearby uninfected bystander cells
stabilizing it and preventing its degradation. ORF61 did show MHC class II expression. With that said, not all
retains NF-κB subunits in the cytoplasm, preventing their cells experience the downregulatory effects of VZV. For
nuclear translocation and subsequent activation of NF-κB- example, while mature MDDCs showed no significant
dependent genes (Figure 2). change in MHC class II expression but exhibited reduced
76
levels of other immune molecules, including MHC class I,
3.3.2. VZV’s effects on MHC class II molecules CD80, CD83, and CD86, opposite effects were noted with
78
In addition, VZV infection impairs IFN-γ-induced monocytes. MHC class I and II proteins have been proven
MHC class II expression by the disruption of the Janus to play an important role in cancer immunosurveillance
kinase transducer and activator of the JAK-STAT signal with a reported MHC class I downregulation in 40 – 90%
transduction pathway. It causes infected cells to be less of human tumors and be associated with a worse prognosis
responsive to cytokine signals. This disruption specifically in disease. 79
inhibits the expression of JAK1, JAK2, and STAT1
proteins, which are critical components of the IFN-γ 4. Reactivation of VZV
signaling pathway. Reduction in protein levels prevents the VZV reactivates from latency in sensory, enteric, and
phosphorylation and subsequent nuclear translocation of other autonomic neurons, leading to HZ. While many
Volume 2 Issue 3 (2025) 20 doi: 10.36922/mi.8320