Page 82 - OR-1-3
P. 82
A C D
B
E F
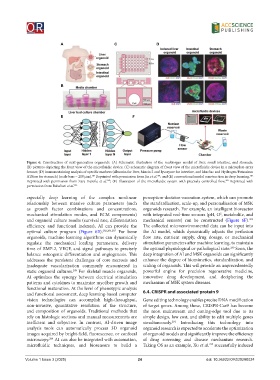
Figure 6. Construction of next-generation organoids. (A) Schematic illustration of the multiorgan model of liver, small intestine, and stomach;
(B) pictures depicting the front view of the microfluidic device; (C) schematic diagram of front view of the microfluidic device in a microplate array
format; (D) immunostaining analysis of specific markers (albumin for liver, Mucin 2 and lysozyme for intestine, and Muc5ac and Hydrogen/Potassium
281
282
ATPase for stomach) (scale bars = 200 μm). Reprinted with permission from Jin et al. ; and (E) conventional model construction in deep learning.
281
282
Reprinted with permission from Zare Harofte et al. ; (F) Illustration of the microfluidic system with precisely controlled flow. Reprinted with
283
permission from Babaliari et al. 283
especially deep learning of the complex nonlinear perception-decision-execution system, which can promote
relationship between massive culture parameters (such the standardization, scale-up, and personalization of MSK
as growth factor combinations and concentrations, organoids research. For example, an intelligent bioreactor
mechanical stimulation modes, and ECM components) with integrated real-time sensors (pH, O , metabolite, and
2
and organoid culture results (survival rate, differentiation mechanical sensors) can be constructed (Figure 6F).
283
efficiency, and functional indexes), AI can provide the The collected microenvironmental data can be input into
optimal culture program (Figure 6E). 282,286,287 For bone the AI model, which dynamically adjusts the perfusion
organoids, machine learning algorithms can dynamically flow rate, nutrient supply, drug dosage, or mechanical
regulate the mechanical loading parameters, delivery stimulation parameters after machine learning, to maintain
time of BMP-2, VEGF, and signal pathways to precisely the optimal physiological or pathological state. Soon, the
290
balance osteogenic differentiation and angiogenesis. This deep integration of AI and MSK organoids can significantly
addresses the persistent challenges of core necrosis and enhance the degree of biomimetics, standardization, and
inadequate vascularization commonly encountered in scaling of organoids. This will provide an unprecedentedly
static organoid cultures. For skeletal muscle organoids, powerful engine for precision regenerative medicine,
288
AI optimizes the synergy between electrical stimulation innovative drug development, and deciphering the
patterns and cytokines to maximize myofiber growth and mechanism of MSK system diseases.
functional maturation. At the level of phenotypic analysis
and functional assessment, deep learning-based computer 6.4. CRISPR and associated protein 9
vision technologies can accomplish high-throughput, Gene editing technology enables precise DNA modification
non-invasive, quantitative resolution of the structure, of target genes. Among these, CRISPR-Cas9 has become
and composition of organoids. Traditional methods that the most mainstream and cutting-edge tool due to its
rely on histologic sections and manual measurements are simple design, low cost, and ability to edit multiple genes
inefficient and subjective. In contrast, AI-driven image simultaneously. Introducing this technology into
291
analysis tools can automatically process 3D organoid organoid research is expected to accelerate the optimization
images acquired by bright-field, fluorescence, or confocal of organoid models and significantly improve the efficiency
microscopy. AI can also be integrated with automation, of drug screening and disease mechanism research.
289
microfluidic techniques, and biosensors to build a Taking OS as an example, Xu et al. successfully induced
292
Volume 1 Issue 3 (2025) 24 doi: 10.36922/OR025280024