Page 26 - TD-3-4
P. 26
Tumor Discovery Expert consensus of NUT carcinoma
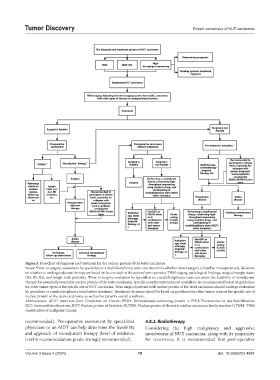
Figure 5. Flowchart of diagnosis and treatment for the nuclear protein of the testis carcinoma
Notes: Prior to surgery, assessment by specialists or a multidisciplinary team can determine whether direct surgery is feasible. Postoperatively, decisions
a
on whether to undergo adjuvant therapy are based on factors such as the patient’s pre-operative TNM staging, pathological findings, surgical margin status
(R0, R1, R2), and lymph node positivity. Prior to surgery, evaluation by specialists or a multidisciplinary team can assess the feasibility of neoadjuvant
b
therapy for potentially resectable nuclear protein of the testis carcinoma. Specific neoadjuvant treatment modalities are recommended based on guidelines
for other tumor types at the specific site of NUT carcinoma. Non-surgical patients with nuclear protein of the testis carcinoma should undergo evaluation
c
by specialists or a multidisciplinary team before treatment. Treatment decisions should be based on guidelines for other tumor types at the specific site of
nuclear protein of the testis carcinoma, as well as the patient’s overall condition.
Abbreviations: AJCC: American Joint Committee on Cancer; BRD4: Bromodomain-containing protein 4; FISH: Fluorescence in situ hybridization;
IHC: Immunohistochemistry; NUT: Nuclear protein of the testis; NUTM1: Nuclear protein of the testis midline carcinoma family member 1; TNM: TNM
classification of malignant tumors.
recommended). Pre-operative assessment by specialized 4.8.2. Radiotherapy
physicians or an MDT can help determine the feasibility Considering the high malignancy and aggressive
and approach of neoadjuvant therapy (level of evidence: invasiveness of NUT carcinoma, along with its propensity
level 4; recommendation grade: strongly recommended). for recurrence, it is recommended that post-operative
Volume 3 Issue 4 (2024) 18 doi: 10.36922/td.4904