Page 31 - TD-4-3
P. 31
Tumor Discovery Immunomodulatory effects of CDK4/6 inhibitors
PD-1/PD-L1 blockade therapies. Conversely, in certain 6. Clinical evidence and trial assessment of
contexts, such as triple-negative breast cancer, CDK4/6 CDK4/6 inhibitors in combination with ICIs
inhibitors may reduce PD-L1 levels through the RB-E2F in HR /HER2 breast cancer
−
+
signaling axis. The overall impact of CDK4/6 inhibition
57
on PD-L1 expression is context-dependent, varying with Ongoing clinical trials are actively exploring the therapeutic
tumor type and microenvironment, highlighting the potential of combining CDK4/6 inhibitors with ICIs for
complex interaction that must be taken into account when patients with HR /HER2 breast cancer. As summarized
+
−
formulating combination treatment strategies. 58 in Table 2, the CheckMate 7A8 trial, which assessed the
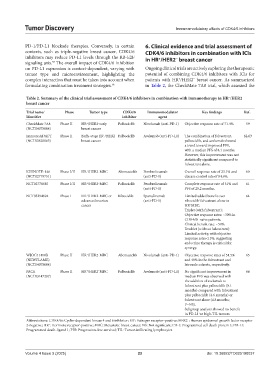
Table 2. Summary of the clinical trial assessment of CDK4/6 inhibitors in combination with immunotherapy in HR /HER2 −
+
breast cancer
Trial name/ Phase Tumor type CDK4/6 Immunomodulator Key findings Ref.
Identifier inhibitor agent
+
−
CheckMate 7A8 Phase II HR /HER2 early Palbociclib Nivolumab (anti-PD-1) Objective response rate of 71.4%. 59
(NCT04075604) breast cancer
−
+
ImmunoADAPT Phase II Early-stage ER /HER2 Palbociclib Avelumab (anti-PD-L1) The combination of fulvestrant, 62,63
(NCT03820063) breast cancer palbociclib, and avelumab showed
a trend toward improved PFS,
with a median PFS of 8.1 months.
However, this improvement was not
statistically significant compared to
fulvestrant alone.
KEYNOTE-146 Phase I/II HR /HER2 MBC Abemaciclib Pembrolizumab Overall response rate of 23.1% and 60
+
−
(NCT02779751) (anti-PD-1) disease control rate of 84.6%.
NCT02778685 Phase I/II HR /HER2 MBC Palbociclib Pembrolizumab Complete response rate of 31% and 61
−
+
(anti-PD-1) PFS of 25.2 months.
NCT03294694 Phase I HR /HER2 MBC or Ribociclib Spartalizumab Limited added benefit over 64
−
+
advanced ovarian (anti-PD-1) ribociclib fulvestrant alone in
+
cancer HR MBC.
+
Triplet (with fulvestrant):
Objective response rates: ~30% in
CDK4/6i-naïve patients.
Clinical benefit rate: ~50%.
Doublet (without fulvestrant):
Limited activity, with objective
response rates<15%, suggesting
endocrine therapy is critical for
synergy.
−
+
WJOG11418B Phase II HR /HER2 MBC Abemaciclib Nivolumab (anti-PD-1) Objective response rates of 54.5% 65
(NEWFLAME) and 40% in the fulvestrant and
(NCT04075604) letrozole cohorts, respectively.
−
+
PACE Phase II HR /HER2 MBC Palbociclib Avelumab (anti-PD-L1) No significant improvement in 66
(NCT03147287) median PFS was observed with
the addition of avelumab to
fulvestrant plus palbociclib (8.1
months) compared with fulvestrant
plus palbociclib (4.6 months) or
fulvestrant alone (4.8 months;
P=NS).
Subgroup analysis showed no benefit
in PD-L1 or high-TIL tumors.
+
Abbreviations: CDK4/6i: Cyclin-dependent kinase 4 and 6 inhibitor; ER : Estrogen receptor-positive; HER2 : Human epidermal growth factor receptor
−
+
+
2-negative; HR : Hormone receptor-positive; MBC: Metastatic breast cancer; NS: Not significant; PD-1: Programmed cell death protein 1; PD-L1:
Programmed death-ligand 1; PFS: Progression-free survival; TIL: Tumor-infiltrating lymphocytes.
Volume 4 Issue 3 (2025) 23 doi: 10.36922/TD025190037