Page 9 - AN-2-3
P. 9
Advanced Neurology Woven Endobridge embolization: Indications and innovation
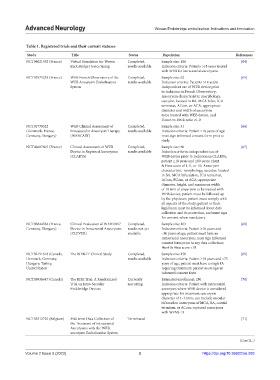
Table 1. Registered trials and their current statuses
Study Title Status Population References
NCT04621552 (France) Virtual Simulation for Woven Completed, Sample size: 186 [64]
EndoBridge Device Sizing results available Inclusion criteria: Patients >18 years treated
with WEB for intracranial aneurysms
NCT01975233 (France) WEB French Observatory of the Completed, Sample size: 62 [65]
WEB Aneurysm Embolization results available Inclusion criteria: Patients >18 years;
System independent use of WEB device prior
to inclusion in French Observatory;
Aneurysm characteristic: morphology,
saccular, located in BA, MCA bifur, ICA
terminus, ACom, or ACA, appropriate
diameter and width of aneurysm
to be treated with WEB device, and
Dome-to-Neck ratio ≥1.0
NCT01778322 WEB Clinical Assessment of Completed, Sample size: 51 [66]
(Denmark, France, Intrasaccular Aneurysm Therapy results available Inclusion criteria: Patient ≥18 years of age;
Germany, Hungary) (WEBCAST) must sign informed consent form prior to
study
NCT02687607 (France) Clinical Assessment of WEB Completed, Sample size: 60 [67]
Device in Ruptured Aneurysms results available Inclusion criteria: independent use of
(CLARYS) WEB device prior to inclusion in CLARYS;
patient ≥18 years and ≤80 years; Hunt
& Hess score of I, II, or III; Aneurysm
characteristic: morphology, saccular, located
in BA, MCA bifurcation, ICA terminus,
ACom, PCom, or ACA; appropriate
diameter, height, and maximum width
of 10 mm of aneurysm to be treated with
WEB device; patient must be followed up
by the physician; patient must comply with
all aspects of the study; patient or their
legal team must be informed about data
collection and its protection, and must sign
for consent when mandatory
NCT03844334 (France, Clinical Evaluation of WEB 0.017 Completed, Sample size: 163 [68]
Germany, Hungary) Device in Intracranial Aneurysms results not yet Inclusion criteria: Patient ≥18 years and
(CLEVER) available <80 years of age; patient must have an
intracranial aneurysm; must sign informed
consent form prior to any data collection;
Hunt & Hess score <III
NCT02191618 (Canada, The WEB-IT Clinical Study Completed, Sample size: 150 [69]
Denmark, Germany, results available Inclusion criteria: Patient ≥18 years and <75
Hungary, Turkey, years of age; patient must have a single IA
United States) requiring treatment; patient must sign an
informed consent form
NCT03936647 (Canada) The RISE Trial: A Randomized Currently Estimated enrollment: 250 [70]
Trial on Intra-Saccular recruiting Inclusion criteria: Patient with intracranial
Endobridge Devices aneurysm where WEB device is considered
appropriate for treatment; aneurysm
diameter of 4–11mm; can include saccular
bifurcation aneurysms of MCA, BA, carotid
terminus, or ACom; ruptured aneurysms
with WFNS ≤3
NCT03312725 (Belgium) Mid-term Data Collection of Terminated - [71]
the Treatment of Intracranial
Aneurysms with the WEB
aneurysm Embolization System
(Cont’d...)
Volume 2 Issue 3 (2023) 3 https://doi.org/10.36922/an.293