Page 86 - AN-3-4
P. 86
Advanced Neurology SARS-CoV-2 in age-associated neurodegeneration
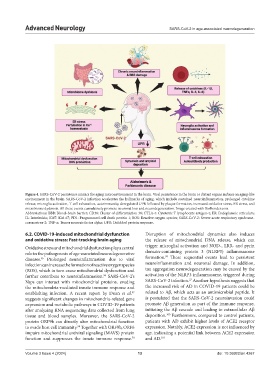
Figure 4. SARS-CoV-2 persistence mimics the aging microenvironment in the brain. Viral persistence in the brain or distant organs induces an aging-like
environment in the brain. SARS-CoV-2 infection accelerates the hallmarks of aging, which include sustained neuroinflammation, prolonged cytokine
release, microglia activation, T-cell exhaustion, autoimmunity, deregulated UPR followed by plaque formation, increased oxidative stress, ER stress, and
microbiome dysbiosis. All these events cumulatively promote neuronal loss and neurodegeneration. Image created with BioRender.com.
Abbreviations: BBB: Blood–brain barrier; CD38: Cluster of differentiation 38; CTLA-4: Cytotoxic T lymphocyte antigen-4; ER: Endoplasmic reticulum;
IL: Interleukin; Ki67: Kiel 67; PD1: Programmed cell death protein 1; ROS: Reactive oxygen species; SARS-CoV-2: Severe acute respiratory syndrome-
coronavirus-2; TNF-α: Tumor necrosis factor alpha; UPR: Unfolded protein response.
6.2. COVID-19-induced mitochondrial dysfunction Disruption of mitochondrial dynamics also induces
and oxidative stress: Fast-tracking brain aging the release of mitochondrial DNA release, which can
Oxidative stress and mitochondrial dysfunction play a central trigger microglial activation and NOD-, LRR- and pyrin
role in the pathogenesis of age-associated neurodegenerative domain-containing protein 3 (NLRP3) inflammasome
99
diseases. Prolonged neuroinflammation due to viral formation. These sequential events lead to persistent
95
infection can increase the formation of reactive oxygen species neuroinflammation and neuronal damage. In addition,
(ROS), which in turn cause mitochondrial dysfunction and tau aggregation neurodegeneration may be caused by the
further contribute to neuroinflammation. SARS-CoV-2’s activation of the NLRP3 inflammasome, triggered during
96
27
Nsps can interact with mitochondrial proteins, evading SARS-CoV-2 infection. Another hypothesis suggests that
the mitochondria-mediated innate immune response and the increased risk of AD in COVID-19 patients could be
97
establishing infection. A recent report by Duan et al. related to Aβ, which acts as an antimicrobial peptide. It
suggests significant changes in mitochondria-related gene is postulated that the SARS-CoV-2 neuroinvasion could
expression and metabolic pathways in COVID-19 patients promote Aβ generation as part of the immune response,
after analyzing RNA sequencing data collected from lung initiating the Aβ cascade and leading to extracellular Aβ
100
tissue and blood samples. Moreover, the SARS-CoV-2 deposition. Furthermore, compared to control patients,
protein ORF9b can directly alter mitochondrial function patients with AD exhibit higher levels of ACE2 receptor
to evade host cell immunity. Together with ORF9b, ORF6 expression. Notably, ACE2 expression is not influenced by
98
impairs mitochondrial antiviral signaling (MAVS) protein age, indicating a potential link between ACE2 expression
function and suppresses the innate immune response. and AD. 101
78
Volume 3 Issue 4 (2024) 13 doi: 10.36922/an.4267