Page 71 - AN-4-3
P. 71
Advanced Neurology Brain regions in olfactory dysfunction in PD
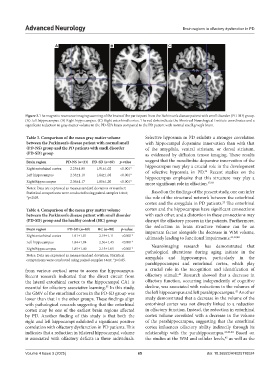
A B C
Figure 2. The magnetic resonance imaging scanning of the brain of the participant from the Parkinson’s disease patient with smell disorder (PD-SD) group.
(A) Left hippocampus. (B) Right hippocampus. (C) Right entorhinal cortex. The red dots indicate the Montreal Neurological Institute coordinates and a
significant reduction in gray matter volume in the PD-SD’s brain compared to the PD patient with normal smell group’s brain.
Table 3. Comparison of the mean gray matter volume Selective hyposmia in PD exhibits a stronger correlation
between the Parkinson’s disease patient with normal smell with hippocampal dopamine innervation than with that
(PD‑NS) group and the PD patients with smell disorder of the amygdala, ventral striatum, or dorsal striatum,
(PD‑SD) group as evidenced by diffusion tensor imaging. These results
Brain region PD‑NS (n=23) PD‑SD (n=69) p‑value suggest that the mesolimbic dopamine innervation of the
Right entorhinal cortex 2.25±1.00 1.91±1.02 <0.001* hippocampus may play a crucial role in the development
of selective hyposmia in PD. Recent studies on the
36
Left hippocampus 2.33±1.10 1.84±1.08 <0.001* hippocampus emphasize that this structure may play a
Right hippocampus 2.36±1.17 1.85±1.20 <0.001* more significant role in olfaction. 37,38
Notes: Data are expressed as mean±standard deviation or number;
Statistical comparisons were conducted using paired-samples t-test; Based on the findings of the present study, one can infer
*p<0.05. the role of the structural network between the entorhinal
cortex and the amygdala in PD patients. The entorhinal
23
Table 4. Comparison of the mean gray matter volume cortex and the hippocampus have significant connections
between the Parkinson’s disease patient with smell disorder with each other, and a distortion in these connections may
(PD‑SD) group and the healthy control (HC) group disrupt the olfactory process in the patients. Furthermore,
the reduction in brain structure volume can be an
Brain region PD‑SD (n=69) HC (n=90) p‑value important factor alongside the decrease in WM volume,
Right entorhinal cortex 1.91±1.01 2.19±1.11 <0.001* ultimately leading to functional impairments. 23,39,40
Left hippocampus 1.84±1.04 2.36±1.45 <0.001*
Neuroimaging research has demonstrated that
Right hippocampus 1.85±1.40 2.15±1.05 <0.001* pathological alterations during aging initiate in the
Notes: Data are expressed as mean±standard deviation; Statistical amygdala and hippocampus, particularly in the
comparisons were conducted using paired-samples t-test; *p<0.05.
parahippocampus and entorhinal cortex, which play
from various cortical areas to access the hippocampus. a crucial role in the recognition and identification of
41
Recent research indicated that the direct circuit from olfactory stimuli. Research showed that a decrease in
the lateral entorhinal cortex to the hippocampal CA1 is olfactory function, occurring independently of cognitive
essential for olfactory associative learning. In this study, decline, was associated with reductions in the volumes of
35
42
the GMV of the entorhinal cortex in the PD-SD group was the left hippocampus and left parahippocampus. Another
lower than that in the other groups. These findings align study demonstrated that a decrease in the volume of the
with pathological research suggesting that the entorhinal entorhinal cortex was not directly linked to a reduction
cortex may be one of the earliest brain regions affected in olfactory function. Instead, the reduction in entorhinal
by PD. Another finding of this study is that both the cortex volume correlated with a decrease in the volume
right and left hippocampi exhibited a significant positive of the parahippocampus, suggesting that the entorhinal
correlation with olfactory dysfunction in PD patients. This cortex influences olfactory ability indirectly through its
indicates that a reduction in bilateral hippocampal volume relationship with the parahippocampus. 41,43,44 Based on
is associated with olfactory deficits in these individuals. the studies at the WM and cellular levels, as well as the
45
Volume 4 Issue 3 (2025) 65 doi: 10.36922/AN025110024