Page 57 - ARNM-2-4
P. 57
Advances in Radiotherapy
& Nuclear Medicine Efficacy of stereotactic radiotherapy
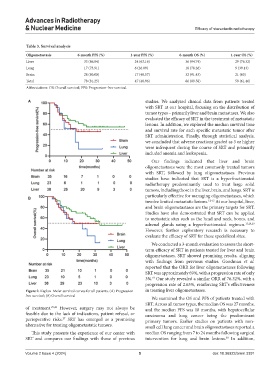
Table 3. Survival analysis
Oligometastasis 6‑month PFS (%) 1‑year PFS (%) 6‑month OS (%) 1‑year OS (%)
Liver 33 (86.84) 24 (63.16) 36 (94.74) 29 (76.32)
Lung 17 (73.91) 6 (26.09) 18 (78.26) 9 (39.13)
Brain 28 (80.00) 17 (48.57) 32 (91.43) 21 (60)
Total 78 (81.25) 47 (48.96) 86 (89.58) 59 (61.46)
Abbreviations: OS: Overall survival; PFS: Progression-free survival.
A studies. We analyzed clinical data from patients treated
with SRT at our hospital, focusing on the distribution of
tumor types – primarily liver and brain metastases. We also
evaluated the efficacy of SRT in the treatment of metastatic
lesions. In addition, we explored the median survival time
and survival rate for each specific metastatic tumor after
SRT administration. Finally, through statistical analysis,
we concluded that adverse reactions graded as 3 or higher
were infrequent during the course of SRT and primarily
included anemia and leukopenia.
Our findings indicated that liver and brain
oligometastases were the most commonly treated tumors
with SRT, followed by lung oligometastases. Previous
studies have indicated that SRT is a hyperfractionated
radiotherapy predominantly used to treat large solid
tumors, including those in the liver, brain, and lungs. SRT is
particularly effective for managing oligometastases, which
B
involve limited metastatic lesions. 12-15 At our hospital, liver,
and brain oligometastases are the primary targets for SRT.
Studies have also demonstrated that SRT can be applied
to metastatic sites such as the head and neck, bones, and
adrenal glands using a hyperfractionated regimen. 15,28,29
However, further exploratory research is necessary to
evaluate the efficacy of SRT for these specialized sites.
We conducted a 3-month evaluation to assess the short-
term efficacy of SRT in patients treated for liver and brain
oligometastases. SRT showed promising results, aligning
with findings from previous studies. Goodman et al.
reported that the ORR for liver oligometastases following
SRT was approximately 69%, with a progression rate of only
19
3%. Our study revealed a similar ORR of 76.32%, with a
progression rate of 2.63%, reinforcing SRT’s effectiveness
Figure 2. Kaplan–Meier survival curves for all patients. (A) Progression- in treating liver oligometastases.
free survival; (B) Overall survival
We examined the OS and PFS of patients treated with
SRT. Across all tumor types, the median OS was 27 months,
of treatment. 25,26 However, surgery may not always be and the median PFS was 18 months, with hepatocellular
feasible due to the lack of indications, patient refusal, or carcinoma and lung cancer being the predominant
perioperative risks. SRT has emerged as a promising primary tumors. Earlier studies on patients with non-
27
alternative for treating oligometastatic tumors. small cell lung cancer and brain oligometastases reported a
This study presents the experience of our center with median OS ranging from 7 to 24 months following surgical
SRT and compares our findings with those of previous intervention for lung and brain lesions. In addition,
30
Volume 2 Issue 4 (2024) 5 doi: 10.36922/arnm.3391