Page 53 - BH-2-4
P. 53
Brain & Heart Stem cells in cardiovascular disease
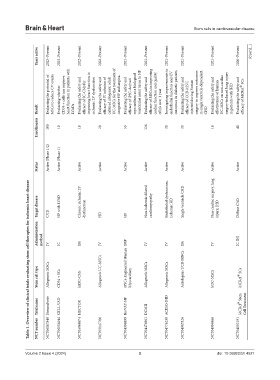
Years active 2023–Present 2022–Present 2022–Present 2022–Present 2021–Present 2021–Present 2021–Present 2021–Present 2021–Present 2020–Present (Cont'd...)
Evaluating the potential of MSCs to reduce CV events Evaluating whether CD34 + cells can improve heart function in patients with Evaluating the safety and efficacy of SC-CMs for improving heart function in ischemic LV dysfunction Evaluating the safety and efficacy of IV infusion of cultured allogeneic adult UC-MSCs for the treatment of congestive HF and angina Evaluating the safety and efficacy of iPSC-derived myocardium as
Result LVADs of life over 1 year CHD
Enrollments 100 10 18 20 53 136 30 30 16 40
Active (Phase 1/2) Active (Phase 1)
Status Active Active Active Active Active Active Active Active
Table 1. Overview of clinical trials evaluating stem cell therapies for ischemic heart disease
Target disease CVD HF with LVAD Chronic ischemic LV dysfunction HD HF Non-ischemic dilated cardiomyopathy Endothelial dysfunction, ischemic HD Single ventricle CHD Non-cardiac surgery, lung injury, IHD Diffuse CAD
Administration method IV IC IM IV IMP IV IV IM IV IC INJ
Stem cell type Allogeneic MSCs CD34 + SCs hESC-CMs Allogeneic UC-MSCs iPSCs-Engineered Human Myocardium Allogeneic MSCs Allogeneic MSCs Autologous UCB-MNCs hUC-MSCs MCRcI® SCs
Trial name StromaForte CELL-VAD HECTOR BioVAT-HF DCMII ACESO-IHD MCRcI® Stem Cell Treatment
NCT number NCT06087848 NCT06154044 NCT05068674 NCT05147766 NCT04396899 NCT04476901 NCT04776239 NCT04907526 NCT04996966 NCT04052191
Volume 2 Issue 4 (2024) 3 doi: 10.36922/bh.4521