Page 55 - BH-2-4
P. 55
Brain & Heart Stem cells in cardiovascular disease
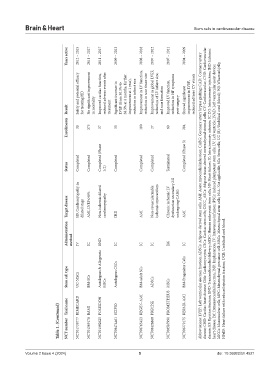
Years active 2012 – 2015 2011 – 2017 2011 – 2017 2009 – 2013 2008 – 2018 2007 – 2012 2007 – 2011 2004 – 2009
Safety and potential efficacy No significant improvement Improved cardiac function, reduced adverse events after Significant increase in LVEF (from 30.3% to 38.5% at 4 months, further improvement at 1 year), reduction in infarct size Improvement in LV function, reduction in scar tissue size Improvement in global LVEF, reduction of LV infarct size, and scar formation Improved LV function, reduction in HF symptoms
Result for treating HD in mortality treatment post-surgery
Enrollments 30 375 37 33 100 27 60 204
Status Completed Completed Completed (Phase 1/2) Completed Completed Completed Terminated Completed (Phase 3)
Target disease HF, Cardiomyopathy in dilated stage AMI, LVEF≤45% Non-ischemic dilated cardiomyopathy IHD AMI Non-revascularizable ischemic myocardium Chronic ischemic LV dysfunction secondary MI undergoing CABG AMI Abbreviations: LVEF: Left ventricular ejection fraction; ADSCs: Adipose-derived stem cells; AMI: Acute myocardial infarction; CABG: Coronary artery bypass grafting; CAD: Coronary artery disease; CHD: Cardiac hear
Administration method IV IC END IC IC IC IM IC
Stem cell type UC-MSCs BM-SCs Autologous & Allogeneic MSCs Autologous CSCs BM-adult SCs ADSCs MSCs BM-Progenitor Cells HFrEF: Heart failure with reduced ejection fraction; UCB: Umbilical cord blood.
Table 1. (Continued) Trial name NCT number RIMECARD NCT01739777 BAMI NCT01569178 POSEIDON NCT01392625 SCIPIO NCT00474461 REGEN-AMI NCT00765453 PRECISE NCT00426868 PROMETHEUS NCT00587990 REPAIR-AMI NCT00279175
Volume 2 Issue 4 (2024) 5 doi: 10.36922/bh.4521