Page 54 - BH-2-4
P. 54
Brain & Heart Stem cells in cardiovascular disease
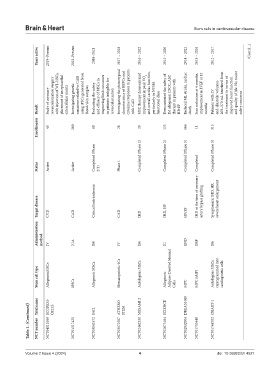
Years active 2019–Present 2012–Present 2018–2021 2017 – 2018 2016 – 2022 2015 – 2020 2014 – 2021 2013 – 2016 2012 – 2017 (Cont'd...)
Study of coronary revascularization surgery with injection of WJ-MSCs, placement of an epicardial extracellular matrix Investigating genetic variations related to CAD using iPSCs generated from blood/skin samples Evaluating the safety and efficacy of MSCs in improving limb outcomes in patients ineligible for revascularization Investigating the phenotypical characteristics of HSPCs and cytokine responses in patients MSC ther
Result with CAD HFrEF death months
Enrollments 40 200 60 28 39 133 566 11 315
Completed (Phase Completed (Phase 2) Completed (Phase 2) Completed (Phase 3) Completed (Phase 3)
Status Active Active 2/3) Phase 1 Completed
Target disease CVD CAD Critical limb ischemia CAD IHD IHD, HF HFrEF IHD at the time of coronary artery bypass grafting Symptomatic IHD, HF, severe heart enlargement
Administration method IV N/A IM IV IM IC END IMP IM
Stem cell type AllogeneicMSCs iPSCs Allogeneic MSCs Hematopoietic SCs Autologous MSCs Allogeneic Adipose-Derived Stromal Cells MPC MPC (iMP) Autologous MSCs, reprogrammed into cardiopoietic cells
Table 1. (Continued) Trial name NCT number SCOREM- NCT04011059 CELLS NCT01517425 SAIL NCT03042572 ATHERO NCT03172507 STEM MESAMI 2 NCT02462330 SCIENCE NCT02673164 DREAM-HF NCT02032004 NCT01753440 CHART-1 NCT01768702
Volume 2 Issue 4 (2024) 4 doi: 10.36922/bh.4521