Page 161 - EJMO-9-1
P. 161
Eurasian Journal of Medicine and
Oncology
Potential of flavonoids against glioblastoma
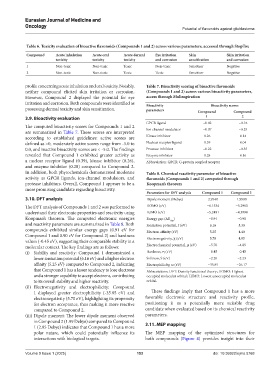
Table 6. Toxicity evaluation of bioactive flavonoids (Compounds 1 and 2) across various parameters, accessed through StopTox
Compound Acute inhalation Acute oral Acute dermal Eye irritation Skin Skin irritation
toxicity toxicity toxicity and corrosion sensitization and corrosion
1 Non-toxic Non-toxic Toxic Non-toxic Sensitizer Negative
2 Non-toxic Non-toxic Toxic Toxic Sensitizer Negative
profile concerning acute inhalation and oral toxicity. Notably, Table 7. Bioactivity scoring of bioactive flavonoids
neither compound elicited skin irritation or corrosion. (Compounds 1 and 2) across various bioactivity parameters,
However, Compound 2 displayed the potential for eye access through Molinspiration
irritation and corrosion. Both compounds were identified as Bioactivity Bioactivity scores
possessing dermal toxicity and skin sensitization. parameters Compound Compound
3.9. Bioactivity evaluation 1 2
GPCR ligand −0.02 −0.16
The computed bioactivity scores for Compounds 1 and 2
are summarized in Table 7. These scores are interpreted Ion channel modulator −0.07 −0.25
according to established guidelines: active scores are Kinase inhibitor 0.26 0.14
defined as >0, moderately active scores range from -5.0 to Nuclear receptor ligand 0.39 0.04
0.0, and inactive bioactivity scores are < -5.0. The findings Protease inhibitor −0.22 −0.35
revealed that Compound 1 exhibited greater activity as Enzyme inhibitor 0.28 0.16
a nuclear receptor ligand (0.39), kinase inhibitor (0.26), Abbreviation: GPCR: G-protein coupled receptor
and enzyme inhibitor (0.28) compared to Compound 2.
In addition, both phytochemicals demonstrated moderate Table 8. Chemical reactivity parameter of bioactive
activity as GPCR ligands, ion-channel modulators, and flavonoids (Compounds 1 and 2) computed through
protease inhibitors. Overall, Compound 1 appears to be a Koopman’s theorem
more promising candidate regarding bioactivity.
Parameters for DFT analysis Compound 1 Compound 2
3.10. DFT analysis Dipole moment (Debye) 2.9540 1.9900
The DFT analysis of Compounds 1 and 2 was performed to HOMO (eV) −6.1534 −5.2983
understand their electronic properties and reactivity using LUMO (eV) −5.2491 −4.3998
Koopman’s theorem. The computed electronic energies Energy gap (ΔE ) −0.91 −0.90
Gap
and reactivity parameters are summarized in Table 8. Both Ionization potential, I (eV) 6.16 5.30
compounds exhibited similar energy gaps (0.91 eV for Electron affinity (eV) 5.25 4.40
Compound 1 and 0.90 eV for Compound 2) and hardness Electronegativity, χ (eV) 5.70 4.85
values (-0.45 eV), suggesting their comparable stability in a
molecular context. The key findings are as follows: Electrochemical potential, µ (eV) −5.70 −4.85
(i) Stability and reactivity: Compound 1 demonstrated a Hardness, η (eV) −0.45 −0.45
lower ionization potential (6.16 eV) and a higher electron Softness, S (eV) −2.20 −2.23
affinity (5.25 eV) compared to Compound 2, indicating Electrophilicity, ω (eV) −35.85 −26.17
that Compound 1 has a lesser tendency to lose electrons Abbreviations: DFT: Density functional theory; HOMO: Highest
and a stronger capability to accept electrons, contributing occupied molecular orbital; LUMO: Lowest unoccupied molecular
to its overall stability and higher reactivity. orbital.
(ii) Electronegativity and electrophilicity: Compound
1 displayed greater electrophilicity (-35.85 eV) and These findings imply that Compound 1 has a more
electronegativity (5.70 eV), highlighting its propensity favorable electronic structure and reactivity profile,
for electron acceptance, thus making it more reactive positioning it as a potentially more suitable drug
compared to Compound 2. candidate when evaluated based on its chemical reactivity
(iii) Dipole moment: The lower dipole moment observed parameters.
in Compound 2 (1.99 Debye) compared to Compound
1 (2.95 Debye) indicates that Compound 1 has a more 3.11. MEP mapping
polar nature, which could potentially influence its The MEP mapping of the optimized structures for
interactions with biological targets. both compounds (Figure 4) provides insight into their
Volume 9 Issue 1 (2025) 153 doi: 10.36922/ejmo.5768