Page 205 - EJMO-9-1
P. 205
Eurasian Journal of Medicine and
Oncology
Cost-effectiveness of nivolumab+chemo for gastric/GEJ cancer
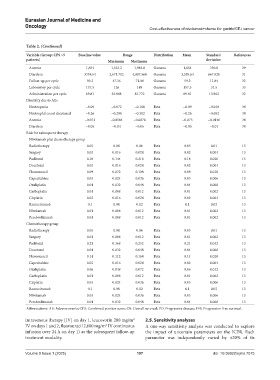
Table 2. (Continued)
Variable (Group: CPS >5 Baseline value Range Distribution Mean Standard References
patients) Minimum Maximum deviation
Anemia 1,654 1,323.2 1,984.8 Gamma 1,654 330.8 29
Diarrhea 3339.64 2,671.712 4,007.568 Gamma 3,339.64 667.928 31
Follow-up per cycle 59.2 47.36 71.04 Gamma 59.2 11.84 32
Laboratory per cycle 157.5 126 189 Gamma 157.5 31.5 33
Administration per cycle 69.81 55.848 83.772 Gamma 69.81 13.962 32
Disutility due to AEs
Neutropenia −0.09 −0.072 −0.108 Beta −0.09 −0.018 34
Neutrophil count decreased −0.26 −0.208 −0.312 Beta −0.26 −0.052 34
Anemia −0.073 −0.0584 −0.0876 Beta −0.073 −0.0146 34
Diarrhea −0.05 −0.04 −0.06 Beta −0.05 −0.01 34
Risk for subsequent therapy
Nivolumab plus chemotherapy group
Radiotherapy 0.05 0.04 0.06 Beta 0.05 0.01 13
Surgery 0.02 0.016 0.024 Beta 0.02 0.004 13
Paclitaxel 0.18 0.144 0.216 Beta 0.18 0.036 13
Docetaxel 0.02 0.016 0.024 Beta 0.02 0.004 13
Fluorouracil 0.09 0.072 0.108 Beta 0.09 0.018 13
Capecitabine 0.03 0.024 0.036 Beta 0.03 0.006 13
Oxaliplatin 0.04 0.032 0.048 Beta 0.04 0.008 13
Carboplatin 0.01 0.008 0.012 Beta 0.01 0.002 13
Cisplatin 0.02 0.016 0.024 Beta 0.02 0.004 13
Ramucirumab 0.1 0.08 0.12 Beta 0.1 0.02 13
Nivolumab 0.01 0.008 0.012 Beta 0.01 0.002 13
Pembrolizumab 0.01 0.008 0.012 Beta 0.01 0.002 13
Chemotherapy group
Radiotherapy 0.05 0.04 0.06 Beta 0.05 0.01 13
Surgery 0.01 0.008 0.012 Beta 0.01 0.002 13
Paclitaxel 0.21 0.168 0.252 Beta 0.21 0.042 13
Docetaxel 0.04 0.032 0.048 Beta 0.04 0.008 13
Fluorouracil 0.14 0.112 0.168 Beta 0.14 0.028 13
Capecitabine 0.02 0.016 0.024 Beta 0.02 0.004 13
Oxaliplatin 0.06 0.048 0.072 Beta 0.06 0.012 13
Carboplatin 0.01 0.008 0.012 Beta 0.01 0.002 13
Cisplatin 0.03 0.024 0.036 Beta 0.03 0.006 13
Ramucirumab 0.1 0.08 0.12 Beta 0.1 0.02 13
Nivolumab 0.03 0.024 0.036 Beta 0.03 0.006 13
Pembrolizumab 0.04 0.032 0.048 Beta 0.04 0.008 13
Abbreviations: AE: Adverse events; CPS: Combined positive score; OS: Overall survival; PD: Progressive disease; PFS: Progression-free survival.
intravenous therapy [IV] on day 1, leucovorin 200 mg/m 2.5. Sensitivity analyses
2
IV on days 1 and 2, fluorouracil 2,600 mg/m IV continuous A one-way sensitivity analysis was conducted to explore
2
infusion over 24 h on day 1) as the subsequent follow-up the impact of uncertain parameters on the ICER. Each
treatment modality. parameter was independently varied by ±20% of its
Volume 9 Issue 1 (2025) 197 doi: 10.36922/ejmo.7075