Page 207 - EJMO-9-1
P. 207
Eurasian Journal of Medicine and
Oncology
Cost-effectiveness of nivolumab+chemo for gastric/GEJ cancer
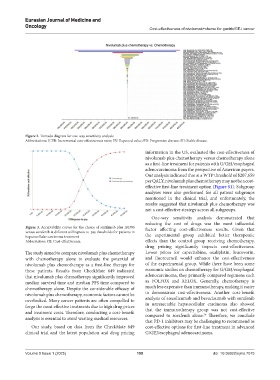
Figure 2. Tornado diagram for one-way sensitivity analysis
Abbreviations: ICER: Incremental cost-effectiveness ratio; EV: Expected value; PD: Progressive disease; SD: Stable disease.
information in the US, evaluated the cost-effectiveness of
nivolumab plus chemotherapy versus chemotherapy alone
as a first-line treatment for patients with G/GEJ/esophageal
adenocarcinoma from the perspective of American payers.
Our analysis indicated that at a WTP threshold of $207,659
per QALY, nivolumab plus chemotherapy may not be a cost-
effective first-line treatment option (Figure S1). Subgroup
analyses were also performed for all patient subgroups
mentioned in the clinical trial, and unfortunately, the
results suggested that nivolumab plus chemotherapy was
not a cost-effective strategy across all subgroups.
One-way sensitivity analysis demonstrated that
reducing the cost of drugs was the most influential
Figure 3. Acceptability curves for the choice of sintilimab plus IBI305 factor affecting cost-effectiveness results. Given that
versus sorafenib at different willingness-to-pay thresholds for patients in
hepatocellular carcinoma treatment the experimental group exhibited better therapeutic
Abbreviation: CE: Cost-effectiveness. effects than the control group receiving chemotherapy,
drug pricing significantly impacts cost-effectiveness.
The study aimed to compare nivolumab plus chemotherapy Lower prices for capecitabine, oxaliplatin, leucovorin,
with chemotherapy alone to evaluate the potential of and fluorouracil would enhance the cost-effectiveness
nivolumab plus chemotherapy as a first-line therapy for of the experimental group. While there have been some
these patients. Results from CheckMate 649 indicated economic studies on chemotherapy for G/GEJ/esophageal
that nivolumab plus chemotherapy significantly improved adenocarcinoma, they primarily compared regimens such
median survival time and median PFS time compared to as FOLFOX and XELOX. Generally, chemotherapy is
chemotherapy alone. Despite the considerable efficacy of much less expensive than immunotherapy, making it easier
nivolumab plus chemotherapy, economic factors cannot be to demonstrate cost-effectiveness. Another cost-benefit
overlooked. Many cancer patients are often compelled to analysis of atezolizumab and bevacizumab with sorafenib
forgo the most effective treatments due to high drug prices in unresectable hepatocellular carcinoma also showed
that the immunotherapy group was not cost-effective
and treatment costs. Therefore, conducting a cost-benefit 36
analysis is essential to avoid wasting medical resources. compared to sorafenib alone. Therefore, we conclude
that PD-1 inhibitors may be challenging to recommend as
Our study, based on data from the CheckMate 649 cost-effective options for first-line treatment in advanced
clinical trial and the latest population and drug pricing G/GEJ/esophageal adenocarcinoma.
Volume 9 Issue 1 (2025) 199 doi: 10.36922/ejmo.7075