Page 208 - EJMO-9-1
P. 208
Eurasian Journal of Medicine and
Oncology
Cost-effectiveness of nivolumab+chemo for gastric/GEJ cancer
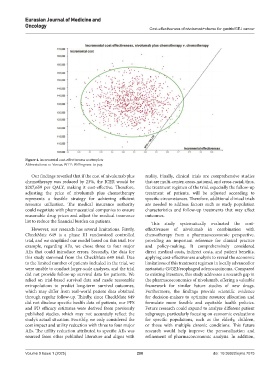
Figure 4. Incremental cost-effectiveness scatterplots
Abbreviations: v.: Versus; WTP: Willingness-to-pay.
Our findings revealed that if the cost of nivolumab plus reality. Finally, clinical trials are comprehensive studies
chemotherapy was reduced by 23%, the ICER would be that are multi-center, cross-national, and cross-racial; thus,
$207,659 per QALY, making it cost-effective. Therefore, the treatment regimen of the trial, especially the follow-up
adjusting the price of nivolumab plus chemotherapy treatment of patients, will be adjusted according to
represents a feasible strategy for achieving efficient specific circumstances. Therefore, additional clinical trials
resource utilization. The medical insurance authority are needed to address factors such as study population
could negotiate with pharmaceutical companies to ensure characteristics and follow-up treatments that may affect
reasonable drug prices and adjust the medical insurance outcomes.
list to reduce the financial burden on patients. This study systematically evaluated the cost-
However, our research has several limitations. Firstly, effectiveness of nivolumab in combination with
CheckMate 649 is a phase III randomized controlled chemotherapy from a pharmacoeconomic perspective,
trial, and we simplified our model based on this trial. For providing an important reference for clinical practice
example, regarding AEs, we chose three to four major and policy-making. It comprehensively considered
AEs that could introduce errors. Secondly, the data for direct medical costs, indirect costs, and patient benefits,
this study stemmed from the CheckMate 649 trial. Due applying cost-effectiveness analysis to reveal the economic
to the limited number of patients included in the trial, we limitations of this treatment regimen in locally advanced or
were unable to conduct larger-scale analyses, and the trial metastatic G/GEJ/esophageal adenocarcinoma. Compared
did not provide follow-up survival data for patients. We to existing literature, this study addresses a research gap in
relied on trial-based survival data and made reasonable the pharmacoeconomics of nivolumab, offering a valuable
extrapolations to predict long-term survival outcomes, framework for similar future studies of new drugs.
which may differ from real-world patient data obtained Furthermore, the findings provide scientific evidence
through regular follow-up. Thirdly, since CheckMate 649 for decision-makers to optimize resource allocation and
did not disclose specific health data of patients, our PFS formulate more feasible and equitable health policies.
and PD efficacy estimates were derived from previously Future research could expand to analyze different patient
published studies, which may not accurately reflect the subgroups, particularly focusing on economic evaluations
study’s actual situation. Fourthly, we only considered the for specific populations, such as the elderly, children,
cost impact and utility reduction with three to four major or those with multiple chronic conditions. This future
AEs. The utility reduction attributed to specific AEs was research would help improve the personalization and
sourced from other published literature and aligns with refinement of pharmacoeconomic analysis. In addition,
Volume 9 Issue 1 (2025) 200 doi: 10.36922/ejmo.7075