Page 38 - GHES-1-2
P. 38
Global Health Econ Sustain Fast-track drug approvals in Brazil
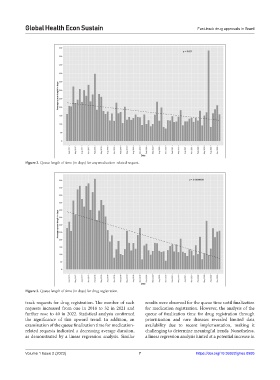
Figure 2. Queue length of time (in days) for any medication-related request.
Figure 3. Queue length of time (in days) for drug registration.
track requests for drug registration. The number of such results were observed for the queue time until finalization
requests increased from one in 2018 to 32 in 2021 and for medication registration. However, the analysis of the
further rose to 40 in 2022. Statistical analysis confirmed queue of finalization time for drug registration through
the significance of this upward trend. In addition, an prioritization and rare diseases revealed limited data
examination of the queue finalization time for medication- availability due to recent implementation, making it
related requests indicated a decreasing average duration, challenging to determine meaningful trends. Nonetheless,
as demonstrated by a linear regression analysis. Similar a linear regression analysis hinted at a potential increase in
Volume 1 Issue 2 (2023) 7 https://doi.org/10.36922/ghes.0995