Page 39 - GHES-1-2
P. 39
Global Health Econ Sustain Fast-track drug approvals in Brazil
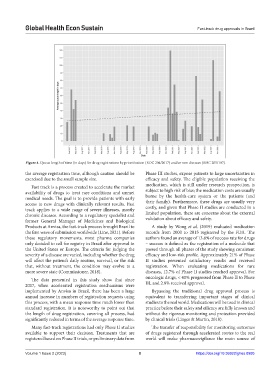
Figure 4. Queue length of time (in days) for drug registrations by prioritization (RDC 204/2017) and/or rare diseases (RDC 205/107).
the average registration time, although caution should be Phase III studies, expose patients to large uncertainties in
exercised due to the small sample size. efficacy and safety. The eligible population receiving the
Fast track is a process created to accelerate the market medication, which is still under research prospection, is
availability of drugs to treat rare conditions and unmet subject to high risk of bias; the medication costs are usually
medical needs. The goal is to provide patients with early borne by the health-care system or the patients (and
access to new drugs with clinically relevant results. Fast their family). Furthermore, these drugs are usually very
track applies to a wide range of severe illnesses, mostly costly, and given that Phase II studies are conducted in a
chronic diseases. According to a regulatory specialist and limited population, there are concerns about the external
former General Manager of Medicines and Biological validation about efficacy and safety.
Products at Anvisa, the fast-track process brought Brazil to A study by Wong et al. (2019) evaluated medication
the first wave of submission worldwide (Line, 2021). Before records from 2000 to 2015 registered by the FDA. The
these regulatory movements, most pharma companies authors found an average of 13.8% of success rate for drugs
only decided to call for registry in Brazil after approval in – success is defined as the registration of a molecule that
the United States or Europe. The criteria for judging the passed through all phases of the study showing consistent
severity of a disease are varied, including whether the drug efficacy and low-risk profile. Approximately 21% of Phase
will affect the patient’s daily routine, survival, or the risk II studies presented satisfactory results and received
that, without treatment, the condition may evolve to a registration. When evaluating medications for rare
more severe state (Commissioner, 2018). diseases, 12.7% of Phase II studies reached approval. For
The data presented in this study show that since oncologic drugs, < 40% progressed from Phase II to Phase
2017, when accelerated registration mechanisms were III, and 2.8% received approval.
implemented by Anvisa in Brazil, there has been a large Bypassing the traditional drug approval process is
annual increase in numbers of registration requests using equivalent to transferring important stages of clinical
this process, with a mean response time much lower than studies to the real world. Medications will be used in clinical
standard registration. It is noteworthy to point out that practice before their safety and efficacy are fully known and
the length of drug registration, covering all process, had without the rigorous monitoring and protection provided
significantly reduced in terms of the average response time. by clinical trials (Linger & Martin, 2018).
Many fast-track registrations had only Phase II studies The transfer of responsibility for monitoring outcomes
available to support their decision. Treatments that are of drugs registered through accelerated routes to the real
registered based on Phase II trials, or preliminary data from world will make pharmacovigilance the main source of
Volume 1 Issue 2 (2023) 8 https://doi.org/10.36922/ghes.0995