Page 36 - GHES-1-2
P. 36
Global Health Econ Sustain Fast-track drug approvals in Brazil
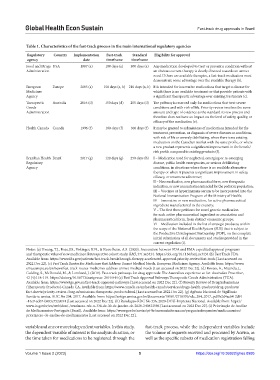
Table 1. Characteristics of the fast‑track process in the main international regulatory agencies
Regulatory Country Implementation Fast‑track Standard Eligibility for approval
agency date timeframe timeframe
Food and Drugs USA 1987 (a) 180 days (a) 300 days (a) Any medication developed to treat or prevent a condition without
Administration an obvious current therapy is clearly directed towards an unmet
need. If there are available therapies, a fast-track medication must
demonstrate some advantage over the available therapy (b).
European Europe 2005 (a) 150 days (a, b) 210 days (a, b) It is intended for innovative medications that target a disease for
Medicines which there is no available treatment or that provide patients with
Agency a significant therapeutic advantage over existing treatments (c).
Therapeutic Australia 2016 (d) 150 days (d) 255 days (d) The pathway is reserved only for medications that treat severe
Goods conditions and with risk of life. Priority review involves the same
Administration amount and type of evidence as the standard review process and
therefore does not have an impact on the level of safety, quality, or
efficacy of the medication (e).
Health Canada Canada 1996 (f) 180 days (f) 300 days (f) It may be granted to submissions of medications intended for the
treatment, prevention, or diagnosis of severe diseases or conditions,
with risk of life or severely debilitating, when there is no existing
medication on the Canadian market with the same profile, or where
a new product represents a significant improvement in the benefit/
risk profile compared to existing products (f).
Brazilian Health Brazil 2017 (g) 120 days (g) 250 days (h) I – Medication used for neglected, emerging or re-emerging
Regulatory disease, public health emergencies, or serious debilitating
Agency conditions, in situations where there is no available alternative
therapy or when it presents a significant improvement in safety,
efficacy, or treatment adherence;
II – New medication, new pharmaceutical form, new therapeutic
indication, or new concentration intended for the pediatric population;
III – Vaccines or hyperimmune serum to be incorporated into the
National Immunization Program of the Ministry of Health;
IV – Innovative or new medication, for active pharmaceutical
ingredient manufactured in the country;
V – The first three petitions for novel generic medication
for each active pharmaceutical ingredient or association and
pharmaceutical form, from distinct economic groups;
VI – Medication included in the list of strategic products, within
the scope of the National Health System (SUS) that is subject to
the Productive Development Partnership (PDP), on the complete
initial submission of all documents and studies provided in the
current regulation (i).
Notes: (a) Hwang, T.J., Ross, J.S., Vokinger, K.N., & Kesselheim, A.S. (2020). Association between FDA and EMA expedited approval programs
and therapeutic value of new medicines: Retrospective cohort study. BMJ, 371:m3434. https://doi.org/10.1136/bmj.m3434 (b) Fast Track FDA.
Available from: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track [Last accessed on
2022 Dec 22]. (c) Fast Track Routes for Medicines that Address Unmet Medical Needs. European Medicines Agency. Available from: https://www.
ema.europa.eu/en/news/fast-track-routes-medicines-address-unmet-medical-needs [Last accessed on 2022 Dec 22]. (d) Bootes, A., Maundu, J.,
Golding, S., McDonald, M., & Lombard, J. (2019). Fast-track pathways for drug approvals: The Australian experience so far. Australian Prescriber,
42 (4):118-119. https://doi.org/10.18773/austprescr. 2019.044 (e) Fast Track Approval Pathways Therapeutic Goods Administration (TGA).
Available from: https://www.tga.gov.au/fast-track-approval-pathways [Last accessed on 2022 Dec 22]. (f) Priority Review of Drug Submissions
(Therapeutic Products)-Canada CA. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/
fact-sheets/priority-review-drug-submissions-therapeutic-products.html [Last accessed on 2022 Dec 22]. (g) Agência Nacional de Vigilância
Sanitária-anvisa. RDC No 204, 2017. Available from: https://antigo.anvisa.gov.br/documents/10181/2718376/rdc_204_2017_pdf/b2d4ae64-2d91
-44e9-ad67-b883c752c094 [Last accessed on 2022 Dec 22]. (h) Resolução-RDC No 336, 2020-DOU-Imprensa Nacional. Available from: https://
www.in.gov.br/en/web/dou/-/resolucao-rdc-n-336-de-30-de-janeiro-de-2020-240823596 [Last accessed on 2022 Dec 22]. (i) Priorização de Análise
de Medicamentos-Português (Brasil). Available from: https://www.gov.br/anvisa/pt-br/acessoainformacao/perguntasfrequentes/medicamentos/
priorizacao-de-analise-de-medicamentos [Last accessed on 2022 Dec 22].
variable and one or more independent variables. In this study, fast-track process, while the independent variables include
the dependent variable of interest is the analysis duration, or the volume of requests received and processed by Anvisa, as
the time taken for medications to be registered through the well as the specific subsets of medication registration falling
Volume 1 Issue 2 (2023) 5 https://doi.org/10.36922/ghes.0995