Page 114 - GHES-2-4
P. 114
Global Health Economics and
Sustainability
Cost-effectiveness of oral semaglutide in Greece
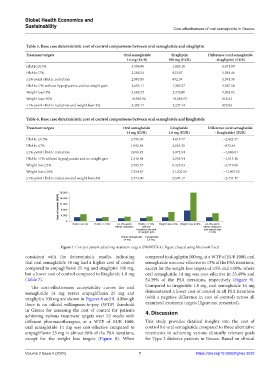
Table 5. Base case deterministic cost of control comparisons between oral semaglutide and sitagliptin
Treatment targets Oral semaglutide Sitagliptin Difference (oral semaglutide
14 mg (EUR) 100 mg (EUR) ‑sitagliptin) (EUR)
HbA1c≤6.5% 3,596.96 1,925.16 1,671.80
HbA1c≤7% 2,206.54 823.07 1,383.46
≥1%-point HbA1c reduction 2,083.95 692.59 1,391.36
HbA1c<7% without hypoglycemia and no weight gain 2,693.11 1,305.57 1,387.54
Weight loss≥5% 3,580.72 2,276.90 1,303.82
Weight loss≥10% 10,965.96 10,349.53 616.42
≥1%-point HbA1c reduction and weight loss≥3% 3,202.17 2,227.14 975.03
Table 6. Base case deterministic cost of control comparisons between oral semaglutide and liraglutide
Treatment targets Oral semaglutide Liraglutide Difference (oral semaglutide
14 mg (EUR) 1.8 mg (EUR) – liraglutide) (EUR)
HbA1c ≤6.5% 2,796.30 4,818.77 −2,022.47
HbA1c ≤7% 1,992.58 2,865.21 −872.64
≥1%-point HbA1c reduction 2,066.83 3,072.84 −1,006.01
HbA1c <7% without hypoglycemia and no weight gain 2,146.84 3,261.94 −1,115.10
Weight loss ≥5% 2,705.37 6,425.03 −3,719.66
Weight loss ≥10% 7,394.67 21,202.59 −13,807.92
≥1%-point HbA1c reduction and weight loss≥3% 2,773.00 5,507.17 −2,734.17
Figure 7. Cost per patient achieving treatment targets (PIONEER 4). Figure created using Microsoft Excel
consistent with the deterministic results, indicating compared to sitagliptin 100 mg, at a WTP of EUR 1000, oral
that oral semaglutide 14 mg had a higher cost of control semaglutide was cost-effective in <5% of the PSA iterations,
compared to empagliflozin 25 mg and sitagliptin 100 mg, except for the weight loss targets of ≥5% and ≥10%, where
but a lower cost of control compared to liraglutide 1.8 mg oral semaglutide 14 mg was cost-effective in 23.49% and
(Table 7). 54.39% of the PSA iterations, respectively (Figure 9).
The cost-effectiveness acceptability curves for oral Compared to liraglutide 1.8 mg, oral semaglutide 14 mg
semaglutide 14 mg versus empagliflozin 25 mg and demonstrated a lower cost of control in all PSA iterations
sitagliptin 100 mg are shown in Figures 8 and 9. Although (with a negative difference in cost of control) across all
there is no official willingness-to-pay (WTP) threshold examined treatment targets (figure not presented).
in Greece for assessing the cost of control for patients 4. Discussion
achieving various treatment targets over 52 weeks with
different pharmacotherapies, at a WTP of EUR 1000, This study provides detailed insights into the cost of
oral semaglutide 14 mg was cost-effective compared to control for oral semaglutide compared to three alternative
empagliflozin 25 mg in almost 90% of the PSA iterations, treatments in achieving various clinically relevant goals
except for the weight loss targets (Figure 8). When for Type 2 diabetes patients in Greece. Based on clinical
Volume 2 Issue 4 (2024) 7 https://doi.org/10.36922/ghes.3032