Page 110 - GHES-2-4
P. 110
Global Health Economics and
Sustainability
Cost-effectiveness of oral semaglutide in Greece
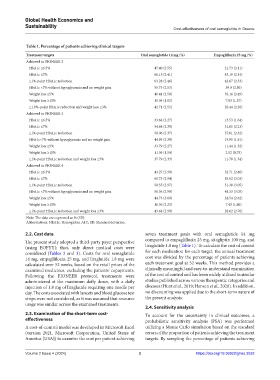
Table 1. Percentage of patients achieving clinical targets
Treatment targets Oral semaglutide 14 mg (%) Empagliflozin 25 mg (%)
Achieved in PIONEER 2
HbA1c ≤6.5% 47.40 (2.55) 21.73 (2.11)
HbA1c ≤7% 66.15 (2.41) 43.19 (2.53)
≥1%-point HbA1c reduction 63.28 (2.46) 42.67 (2.53)
HbA1c <7% without hypoglycemia and no weight gain 55.73 (2.53) 39.0 (2.50)
Weight loss ≥5% 40.41 (2.50) 39.16 (2.49)
Weight loss ≥10% 15.03 (1.82) 7.83 (1.37)
≥1.0%-point HbA1c reduction and weight loss ≥3% 42.71 (2.52) 26.44 (2.26)
Achieved in PIONEER 3
HbA1c ≤6.5% 33.64 (2.27) 13.53 (1.64)
HbA1c ≤7% 54.84 (2.39) 31.65 (2.23)
≥1%-point HbA1c reduction 58.06 (2.37) 37.61 (2.32)
HbA1c<7% without hypoglycemia and no weight gain 44.93 (2.39) 19.95 (1.91)
Weight loss ≥5% 33.79 (2.27) 11.44 (1.52)
Weight loss ≥10% 11.03 (1.50) 2.52 (0.75)
≥1%-point HbA1c reduction and weight loss ≥3% 37.79 (2.33) 11.70 (1.54)
Achieved in PIONEER 4
HbA1c ≤6.5% 43.27 (2.99) 32.71 (2.86)
HbA1c ≤7% 60.73 (2.94) 55.02 (3.03)
≥1%-point HbA1c reduction 58.55 (2.97) 51.30 (3.05)
HbA1c <7% without hypoglycemia and no weight gain 56.36 (2.99) 48.33 (3.05)
Weight loss ≥5% 44.73 (3.00) 24.54 (2.62)
Weight loss ≥10% 16.36 (2.23) 7.43 (1.60)
≥1%-point HbA1c reduction and weight loss ≥3% 43.64 (2.99) 28.62 (2.76)
Note: The data are expressed as % (SD).
Abbreviations: HbA1c: Hemoglobin A1C; SD: Standard deviation.
2.2. Cost data seven treatment goals with oral semaglutide 14 mg
The present study adopted a third-party payer perspective compared to empagliflozin 25 mg, sitagliptin 100 mg, and
(using EOPYY); thus, only direct medical costs were liraglutide 1.8 mg (Table 1). To calculate the cost of control
considered (Tables 2 and 3). Costs for oral semaglutide for each medication for each target, the annual treatment
14 mg, empagliflozin 25 mg, and liraglutide 1.8 mg were cost was divided by the percentage of patients achieving
calculated over 52 weeks, based on the retail prices of the each treatment goal at 52 weeks. This method provides a
examined medicines, excluding the patients’ copayments. clinically meaningful and easy-to-understand examination
Following the PIONEER protocol, treatments were of the cost of control and has been widely utilized in similar
administered at the maximum daily doses, with a daily studies published across various therapeutic categories and
injection of 1.8 mg of liraglutide requiring one needle per diseases (Hunt et al., 2019; Hansen et al., 2020). In addition,
day. The costs associated with lancets and blood glucose test no discounting was applied due to the short-term nature of
strips were not considered, as it was assumed that resource the present analysis.
usage was similar across the examined treatments.
2.4. Sensitivity analysis
2.3. Examination of the short-term cost- To account for the uncertainty in clinical outcomes, a
effectiveness probabilistic sensitivity analysis (PSA) was performed
A cost-of-control model was developed in Microsoft Excel utilizing a Monte Carlo simulation based on the standard
(version 2021, Microsoft Corporation, United States of errors of the proportion of patients achieving the treatment
America [USA]) to examine the cost per patient achieving targets. By sampling the percentage of patients achieving
Volume 2 Issue 4 (2024) 3 https://doi.org/10.36922/ghes.3032