Page 111 - GHES-2-4
P. 111
Global Health Economics and
Sustainability
Cost-effectiveness of oral semaglutide in Greece
Table 2. Drug acquisition costs
Cost of interventions Pharmacy selling Copayment (%) Payer cost Payer cost per Reference
price (EUR) (EUR) day (EUR)
Oral semaglutide 14 mg 110.43 10 99.39 3.31 Ministerial decree
Empagliflozin 25 mg 48.19 10 43.37 1.45 (67328/December 29, 2023)
Sitagliptin 100 mg 22.19 10 19.97 0.71
Liraglutide 1.8 mg 93.87 10 84.48 4.22
Table 3. Consumables cost
Consumable costs Reimbursed Co‑payment EOPYY cost EOPYY Reference
price cost/needle
NOVOFINE 32G 0.23/0.25×6 mm×100 units 9.18 0% 9.19 0.09 Government Gazzete
(FEK B’ 4045/November 17, 2017)
Note: EOPYY: Greek third-party payer.
treatment targets, the cost of control for each medication
was calculated and repeated 10,000 times. From these
iterations, the average cost of control for each medication
was determined, along with the 95% confidence interval,
using the percentile method.
3. Results
3.1. Annual treatment costs
The annual treatment costs for oral semaglutide 14 mg,
empagliflozin 25 mg, and sitagliptin 100 mg were estimated
to be EUR 1,210.04, EUR 528.04, and EUR 260.51,
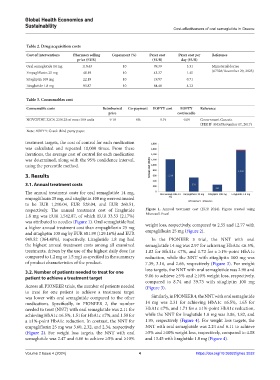
respectively. The annual treatment cost of liraglutide Figure 1. Annual treatment cost (EUR 2024). Figure created using
1.8 mg was EUR 1,542.87, of which EUR 33.53 (2.17%) Microsoft Excel
was attributed to needles (Figure 1). Oral semaglutide had weight loss, respectively, compared to 2.55 and 12.77 with
a higher annual treatment cost than empagliflozin 25 mg
and sitagliptin 100 mg by EUR 681.99 (129.16%) and EUR empagliflozin 25 mg (Figure 2).
949.52 (364.48%), respectively. Liraglutide 1.8 mg had In the PIONEER 3 trial, the NNT with oral
the highest annual treatment costs among all examined semaglutide 14 mg was 2.97 for achieving HbA1c ≤6.5%,
treatments, driven by the use of the highest daily dose (as 1.82 for HbA1c ≤7%, and 1.72 for a ≥1%-point HbA1c
compared to 1.2 mg or 1.5 mg) as specified in the summary reduction, while the NNT with sitagliptin 100 mg was
of product characteristics of the product. 7.39, 3.16, and 2.66, respectively (Figure 3). For weight
loss targets, the NNT with oral semaglutide was 2.96 and
3.2. Number of patients needed to treat for one
patient to achieve a treatment target 9.06 to achieve ≥5% and ≥10% weight loss, respectively,
compared to 8.74 and 39.73 with sitagliptin 100 mg
Across all PIONEER trials, the number of patients needed (Figure 3).
to treat for one patient to achieve a treatment target
was lower with oral semaglutide compared to the other Similarly, in PIONEER 4, the NNT with oral semaglutide
medications. Specifically, in PIONEER 2, the number 14 mg was 2.31 for achieving HbA1c ≤6.5%, 1.65 for
needed to treat (NNT) with oral semaglutide was 2.11 for HbA1c ≤7%, and 1.71 for a ≥1%-point HbA1c reduction,
achieving HbA1c ≤6.5%, 1.51 for HbA1c ≤7%, and 1.58 for while the NNT for liraglutide 1.8 mg was 3.06, 1.82, and
a ≥1%-point HbA1c reduction. In contrast, the NNT for 1.95, respectively (Figure 4). For weight loss targets, the
empagliflozin 25 mg was 3.60, 2.32, and 2.34, respectively NNT with oral semaglutide was 2.24 and 6.11 to achieve
(Figure 2). For weight loss targets, the NNT with oral ≥5% and ≥10% weight loss, respectively, compared to 4.08
semaglutide was 2.47 and 6.66 to achieve ≥5% and ≥10% and 13.45 with liraglutide 1.8 mg (Figure 4).
Volume 2 Issue 4 (2024) 4 https://doi.org/10.36922/ghes.3032