Page 36 - GPD-2-4
P. 36
Gene & Protein in Disease Cyanine and cancer therapy
3.2. Pro-apoptotic proteins
3.2.1. BAX and BAK
BAX and BAK, pro-apoptotic members of the Bcl-2
family, cause MOMP by forming large pores in the outer
mitochondrial membrane through conformational changes
and oligomerization [91,92] . Dysregulation of BAX, often
through suppression or mutation, is commonly observed
in cancer, with reduced expression of BAX associated with
poorer prognosis [93,94] . In addition, deficiency in BAK, along
with BAX, significantly inhibits mitochondrial-mediated
apoptotic cell death . Studies have identified pro-apoptotic
[95]
BAX mutations in colorectal cancer (CRC), which contribute
to resistance against anti-cancer therapies . Alterations
[96]
in the BCL-2/BAX ratio have also been implicated in
chronic lymphocytic leukemia . Furthermore, intrinsic
[97]
pathways can be disrupted in cancer through mechanisms
such as reduced expression of Apaf-1in melanoma due to
abnormalities in promoter methylation [98,99] .
3.2.2. Cytochrome C
Cyto C, located within the mitochondrial, is a small
spherical core-coding protein containing covalently
linked heme groups. Studies on Cyto C-deficient BALB/C
nude mice have revealed that these mice die during mid-
gestation, suggesting that Cyto C is a protein essential for
organism development and mitochondrial adenosine
triphosphate (ATP) production [100] . Functioning as a
crucial signaling molecule, Cyto C initiates apoptosis by
activating downstream cysteine cascades. The BAX/BAK
pore mediates the release of Cyto C and mitochondrial DNA
(mtDNA) from the mitochondria . Moreover, Cyto C plays
[39]
an irreplaceable role in ATP generation and cell survival.
Through its interaction with Apaf-1, Cyto-C forms an active
apoptosome, thereby initiating caspase-9 activity and the
downstream caspase cascade. Furthermore, Cyto C plays a
vital role in both the clearance and generation of ROS [101-103] .
In summary, Cyto C is an integral component in apoptosis.
3.2.3. Caspase family
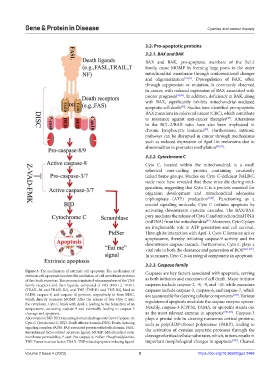
Figure 3. The mechanism of extrinsic cell apoptosis. The mechanism of Caspases are key factors associated with apoptosis, serving
extrinsic cell apoptosis involves the mediation of cell membrane proteins
of the death receptors. This process is initiated when members of the TNF as both initiators and executors of cell death. Major initiator
family receptors and their ligands, composed of FAS (FAS-L), TRAIL caspases include caspase-2, -8, -9, and -10, while executors
(TRAIL-R1 and TRAIL-R2), and TNF (TNF-R1 and TNF-R2), bind to caspases include caspase-3, caspase-6, and caspase-7, which
FADD, caspase-8, and caspase-10 proteins, respectively, to form DISC, are accountable for cleaving cellular components [104] . Various
which directly increases MOMP. After the release of free Cyto C into regulators of apoptosis modulate the caspase enzyme system.
the cytoplasm, Cyto C binds with Apaf-1, leading to the formation of an
apoptosome, activating caspase-9 and eventually leading to caspase-3 Notably, caspase-3 (CPP32, YAMA, or apopain) stands out
cleavage and apoptosis. as the most relevant enzyme in apoptosis [105,106] . Caspase-3
Abbreviations: BID: BH3 interacting domain death agonist; Cas10: Caspase-10; plays a pivotal role in cleaving numerous critical proteins,
Cyto C: Cytochrome C; DED: Death effector domain; DISC: Death-inducing such as poly(ADP-ribose) polymerase (PARP), leading to
signaling complex;FADD: FAS-associated protein with death domain; FASL: the activation of cysteine aspartate proteases through the
Recombinant factor-related apoptosis ligand; MOMP: Mitochondrial outer
membrane permeability; P-cas8: Pro-caspase-8; PtdSer: Phosphatidylserine; cleavage of critical cellular substrates, which, in turn, results in
TNF: Tumor necrosis factor; TRAIL: TNF-related apoptosis-inducing ligand. important morphological changes in apoptosis [107] . Cleaved
Volume 2 Issue 4 (2023) 5 https://doi.org/10.36922/gpd.2486