Page 68 - GPD-2-4
P. 68
Gene & Protein in Disease Topical Me-EGF application in melanoma tumor growth
Figure 1. Effect of Me-EGF and its ingredients on the viability of B16-F10 melanoma cells. Cell viability was determined by MTT assay. Data from triplicate
experiments are expressed as mean ± SD; data of the untreated control group are expressed as 100%. ns: Not significant.
A B
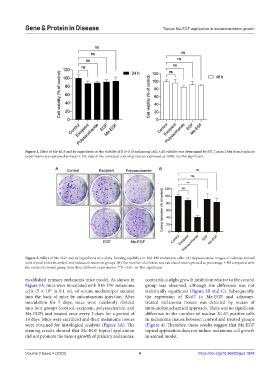
Figure 2. Effect of Me-EGF and its ingredients on colony-forming capability in B16-F10 melanoma cells. (A) Representative images of colonies stained
with crystal violet in control and indicated treatment groups. (B) The number of colonies was calculated and expressed as percentage ± SD compared with
the results of control group from three different experiments. **P < 0.01, ns: Not significant.
established primary melanoma mice model. As shown in contrarily, a slight growth inhibition relative to the control
Figure 3A, mice were inoculated with B16-F10 melanoma group was observed, although the difference was not
cells (5 × 10 in 0.1 mL of serum medium/per mouse) statistically significant (Figure 3B and C). Subsequently,
5
into the back of mice by subcutaneous injection. After the expression of Ki-67 in Me-EGF and adjuvant-
inoculation for 7 days, mice were randomly divided treated melanoma tissues was detected by means of
into four groups (control, excipient, polysaccharide, and immunohistochemical approach. There was no significant
Me-EGF) and treated once every 2 days for a period of difference in the number of nuclear Ki-67-positive cells
14 days. Mice were sacrificed and their melanoma tissues in melanoma tissues between control and treated groups
were obtained for histological analysis (Figure 3A). The (Figure 4). Therefore, these results suggest that Me-EGF
staining results showed that Me-EGF topical application topical application does not induce melanoma cell growth
did not promote the tumor growth of primary melanoma; in animal model.
Volume 2 Issue 4 (2023) 4 https://doi.org/10.36922/gpd.1848