Page 69 - GPD-2-4
P. 69
Gene & Protein in Disease Topical Me-EGF application in melanoma tumor growth
A
B C
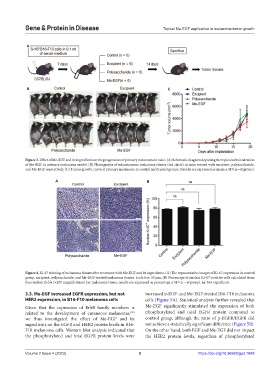
Figure 3. Effect of Me-EGF and its ingredients on the progression of primary melanoma in mice. (A) Schematic diagram depicting the topical administration
of Me-EGF in primary melanoma model. (B) Photographs of subcutaneous melanoma tissues (red circle) in mice treated with excipient, polysaccharide,
and Me-EGF, respectively. (C) Tumor growth curve of primary melanoma in control and treated groups. Results are expressed as mean ± SD (n = 6/group).
A B
Figure 4. Ki-67 staining of melanoma tissues after treatment with Me-EGF and its ingredients. (A) The representative images of Ki-67 expression in control
group, excipient, polysaccharide, and Me-EGF-treated melanoma tissues. Scale bar: 50 µm. (B) Percentage of nuclear Ki-67-positive cells calculated from
five random fields (×200 magnification) per melanoma tissue; results are expressed as percentage ± SD (n = 6/group). ns: Not significant.
3.3. Me-EGF increased EGFR expression, but not increased in EGF- and Me-EGF-treated B16-F10 melanoma
HER2 expression, in B16-F10 melanoma cells cells (Figure 5A). Statistical analysis further revealed that
Given that the expression of ErbB family members is Me-EGF significantly stimulated the expression of both
related to the development of cutaneous melanoma, phosphorylated and total EGFR protein compared to
[19]
we thus investigated the effect of Me-EGF and its control group, although the ratio of p-EGFR/EGFR did
ingredients on the EGFR and HER2 protein levels in B16- not achieve a statistically significant difference (Figure 5B).
F10 melanoma cells. Western blot analysis indicated that On the other hand, both EGF and Me-EGF did not impact
the phosphorylated and total EGFR protein levels were the HER2 protein levels, regardless of phosphorylated
Volume 2 Issue 4 (2023) 5 https://doi.org/10.36922/gpd.1848