Page 36 - GTM-3-1
P. 36
Global Translational Medicine Immune response in humans due to COVID-19 infection
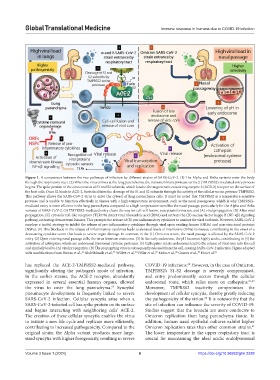
Figure 1. A comparison between the two pathways of infection by different strains of SARS-CoV-2. (1) The Alpha and Delta variants enter the body
through the respiratory tract. (2) When the virus arrives at the lung parenchyma, the transmembrane protease serine 2 (TMPRSS2)-mediated entry process
begins. The spike protein of the virus consists of S1 and S2 subunits, which bind to the angiotensin-converting enzyme 2 (ACE-2) receptor on the surface of
the host cells. Once S2 binds to ACE-2, furin facilitates the cleavage of the S1 and S2 subunits through the activity of the cellular serine protease TMPRSS2.
This pathway allows the SARS-CoV-2 virus to enter the cytosol of lung parenchyma cells. It must be noted that TMPRSS2 is a temperature-sensitive
protease and is unable to function effectively in tissues with a high-temperature environment, such as the nasal passageway, which is why TMPRSS2-
mediated entry is more efficient in the lung parenchyma compared to a high-temperature zone like the nasal passage, particularly for the Alpha and Delta
variants of SARS-CoV-2. (3) TMPRSS2-mediated entry clears the way for cell-cell fusion, syncytium formation, and (A) viral propagation. (B) After viral
propagation, (C) cytosolic toll-like receptors (TLR7/8) detect viral ribonucleic acid (RNA) and activate the (D) nuclear factor kappa B (NF-κβ) signaling
pathway, activating downstream kinases. This prompts the release of (E) pro-inflammatory cytokines to combat the viral outbreak. However, SARS-CoV-2
employs a tactful strategy to inhibit the release of pro-inflammatory cytokines through viral open reading frames (ORFs) and non-structural proteins
(NSPs). (F) This blockade in the release of inflammatory cytokines leads to elevated levels of interferons (IFNs) in tissues, contributing to the onset of a
devastating cytokine storm that leads to severe organ damage. In contrast, in the (1) Omicron strain, the nasal passage is affected by the SARS-CoV-2
entry. (2) Upon entering nasal passage cells, the virus forms an endosome. (3) In the early endosome, the pH becomes highly acidic, contributing to (4) the
activation of cathepsins, which are endosomal lysosomal cysteine proteases. (5) Cathepsins in late endosomes lead to the release of viral core into the cell
and similarly lead to (A) viral propagation. (B) The propagating virus is subsequently released from the cell, causing SARS-CoV-2 infection. Figure adapted
65
66
63
64
67
with modifications from Biswas et al., AbdelMassih et al., Willett et al., Pišlar et al., Kubo et al., Gomes et al., Hui et al. 61
21
has replaced the ACE-2-TMPRSS2-mediated pathway, COVID-19 infections. However, in the case of Omicron,
60
significantly altering the pathogen’s mode of infection. TMPRSS2’s S1-S2 cleavage is severely compromised,
In the earlier strains, the ACE-2 receptor, abundantly and entry predominantly occurs through the cellular
expressed in several essential human organs, allowed endosomal route, which relies more on cathepsins. 61,62
the virus to enter the lung parenchyma. Syncytial Moreover, TMPRSS2 inactivity compromises the
59
pneumocyte development is frequently linked to severe development of cellular syncytia, thereby greatly reducing
62
SARS-CoV-2 infection. Cellular syncytia arise when a the pathogenicity of the virion. It is noteworthy that the
SARS-CoV-2-infected cell has spike protein on its surface site of infection can influence the severity of COVID-19.
and begins interacting with neighboring cells’ ACE-2. Studies suggest that the bronchi are more conducive to
The creation of these cellular syncytia enables the virus Omicron replication than lung parenchyma tissue. In
to initiate a new life cycle and replicate more efficiently, addition, human nasal epithelial cultures exhibit higher
contributing to increased pathogenicity. Compared to the Omicron replication rates than other common strains.
61
original strain, the Alpha variant produces more large- The lower temperature in the upper respiratory tract is
sized syncytia with higher fusogenicity, resulting in severe crucial for maintaining the ideal acidic endolysosomal
Volume 3 Issue 1 (2024) 6 https://doi.org/10.36922/gtm.2228