Page 12 - GTM-3-2
P. 12
Global Translational Medicine Mitochondria and ferroptotic cell death
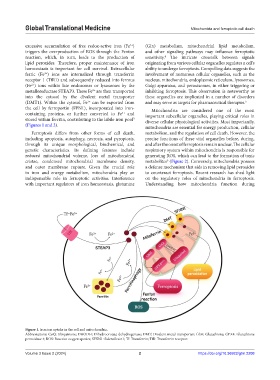
excessive accumulation of free redox-active iron (Fe ) (Gln) metabolism, mitochondrial lipid metabolism,
2+
triggers the overproduction of ROS through the Fenton and other signaling pathways may influence ferroptotic
reaction, which, in turn, leads to the production of sensitivity. The intricate crosstalk between signals
4
lipid peroxides. Therefore, proper maintenance of iron originating from various cellular organelles regulates a cell’s
homeostasis is important for cell survival. Extracellular ability to undergo ferroptosis. Compelling data suggests the
ferric (Fe ) ions are internalized through transferrin involvement of numerous cellular organelles, such as the
3+
receptor 1 (TfR1) and subsequently reduced into ferrous nucleus, mitochondria, endoplasmic reticulum, lysosomes,
(Fe ) ions within late endosomes or lysosomes by the Golgi apparatus, and peroxisomes, in either triggering or
2+
metalloreductase STEAP3. These Fe are then transported inhibiting ferroptosis. This observation is noteworthy as
2+
into the cytosol by the divalent metal transporter these organelles are implicated in a number of disorders
(DMT1). Within the cytosol, Fe can be exported from and may serve as targets for pharmaceutical therapies. 5
2+
the cell by ferroportin (FPN1), incorporated into iron- Mitochondria are considered one of the most
containing proteins, or further converted to Fe and important subcellular organelles, playing critical roles in
3+
stored within ferritin, contributing to the labile iron pool diverse cellular physiological activities. Most importantly,
3
(Figures 1 and 2). mitochondria are essential for energy production, cellular
Ferroptosis differs from other forms of cell death, metabolism, and the regulation of cell death. However, the
including apoptosis, autophagy, necrosis, and pyroptosis, precise functions of these vital organelles before, during,
through its unique morphological, biochemical, and and after the onset of ferroptosis remain unclear. The cellular
genetic characteristics. Its defining features include respiratory system within mitochondria is responsible for
reduced mitochondrial volume, loss of mitochondrial generating ROS, which can lead to the formation of toxic
cristae, condensed mitochondrial membrane density, metabolites (Figure 2). Conversely, mitochondria possess
6
and outer membrane rupture. Given the crucial role a defense mechanism that aids in removing lipid peroxides
in iron and energy metabolism, mitochondria play an to counteract ferroptosis. Recent research has shed light
indispensable role in ferroptotic activities. Interference on the regulatory roles of mitochondria in ferroptosis.
with important regulators of iron homeostasis, glutamine Understanding how mitochondria function during
Figure 1. Iron ion uptake in the cell and mitochondria.
Abbreviations: CoQ: Ubiquinone; DHODH: Dihydroorotate dehydrogenase; DMT: Divalent metal transporter; GSH: Glutathione; GPX4: Glutathione
peroxidase 4; ROS: Reactive oxygen species; SFXN1: Sideroflexin1; Tf: Transferrin; TfR: Transferrin receptor.
Volume 3 Issue 2 (2024) 2 https://doi.org/10.36922/gtm.2208