Page 104 - GTM-3-4
P. 104
Global Translational Medicine Prediction of in-stent restenosis
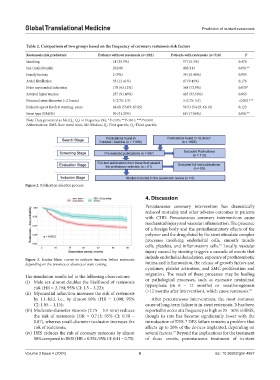
Table 2. Comparison of two groups based on the frequency of coronary restenosis risk factors
Restenosis risk predictors Patients without restenosis (n=282) Patients with restenosis (n=516) P
Smoking 14 (25.9%) 57 (15.3%) 0.476
Sex (male/female) 202/80 402/113 0.001**
Family history 2 (9%) 39 (10.48%) 0.993
Atrial fibrillation 35 (12.41%) 47 (9.49%) 0.176
Prior myocardial infarction 178 (63.12%) 368 (73.9%) 0.033*
Arterial hypertension 257 (91.46%) 465 (93.56%) 0.065
Nominal stent diameter (<2.5 mm) 3 (2.75; 3.5) 3 (2.75; 3.5) <0.001***
Patient’s age at the first stenting, years 61.68 (55.09; 67.05) 59.53 (54.29; 66.19) 0.123
Stent type (BMS%) 90 (31.25%) 68 (17.66%) 0.001**
Note: Data presented as Me (Q ; Q ) or frequency (%); *P<0.05; **P<0.01; ***P<0.001.
3
1
Abbreviations: BMS: Bare metal stent; Me: Median; Q : First quartile; Q : Third quartile.
1
3
Figure 2. Publication selection process
4. Discussion
Percutaneous coronary intervention has dramatically
reduced mortality and other adverse outcomes in patients
with CHD. Percutaneous coronary interventions cause
mechanical injury and vascular inflammation. The presence
of a foreign body and the proinflammatory effects of the
polymer and the drug eluted by the stent stimulate complex
processes involving endothelial cells, smooth muscle
cells, platelets, and inflammatory cells. Locally, vascular
14
injury caused by stenting triggers a cascade of events that
include endothelial denudation, exposure of prothrombotic
Figure 3. Kaplan-Meier curves to evaluate function before restenosis
depending on the presence or absence of stent coating. intima and inflammation, the release of growth factors and
cytokines, platelet activation, and SMC proliferation and
The simulation results led to the following observations: migration. The result of these processes may be healing
(i) Male sex almost doubles the likelihood of restenosis or pathological processes, such as excessive neointimal
risk (HR = 2.194; 95% CI: 1.5 – 3.22); hyperplasia (in 6 – 12 months) or neoatherogenesis
15
(ii) Myocardial infarction increases the risk of restenosis (>12 months after intervention), which cause restenosis.
by 1.1-fold, i.e., by almost 10% (HR = 1.098; 95% After percutaneous interventions, the most common
CI: 1.05 – 1.15); cause of long-term failure is in-stent restenosis. It has been
(iii) Moderate-diameter stenosis (2.75 – 3.5 mm) reduces reported to occur at a frequency as high as 25 – 50% in BMS,
the risk of restenosis (HR = 0.713; 95% CI: 0.58 – though its rate has become significantly lower with the
0.87), whereas small-diameter occlusion increases the introduction of DES. DES failure remains a problem that
16
risk of restenosis; affects up to 20% of the devices implanted, depending on
(iv) DES reduces the risk of coronary restenosis by almost several factors. Beyond the implications for the treatment
17
50% compared to BMS (HR = 0.554; 95% CI: 0.41 – 0.75). of these events, percutaneous treatment of in-stent
Volume 3 Issue 4 (2024) 6 doi: 10.36922/gtm.4957