Page 142 - IJB-10-1
P. 142
International Journal of Bioprinting Bioprinting organoids for toxicity testing
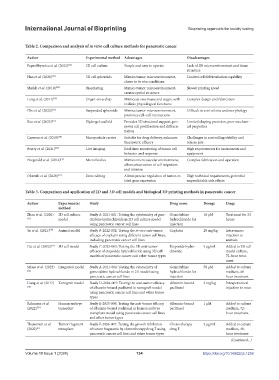
Table 2. Comparison and analysis of in vitro cell culture methods for pancreatic cancer
Author Experimental method Advantages Disadvantages
Papaefthymiou et al. (2022) [22] 2D cell culture Simple and easy to operate Lack of 3D microenvironment and tissue
structure
Han et al. (2020) [23] 3D cell spheroids Mimics tumor microenvironment, Limited cell differentiation capability
closer to in vivo conditions
Sheikh et al. (2010) [24] Bioprinting Mimics tumor microenvironment, Slower printing speed
creates spatial structure
Long et al. (2011) [25] Organ-on-a-chip Mimics in vivo tissue and organ, with Complex design and fabrication
realistic physiological functions
Cho et al. (2023) [26] Suspended spheroids Mimics tumor microenvironment, Difficult to control size and morphology
promotes cell–cell interactions
Kuo et al. (2023) [27] Hydrogel scaffold Provides 3D structural support, pro- Limited shaping precision, poor mechani-
motes cell proliferation and differen- cal properties
tiation
Capurso et al. (2019) [28] Nanoparticle carrier Suitable for drug delivery, enhances Challenges in controlling stability and
therapeutic efficacy release rate
Beatty et al. (2021) [29] Live imaging Real-time monitoring of tumor cell High requirements for instruments and
behavior and response equipment
Fitzgerald et al. (2014) [31] Microfluidics Mimics microvascular environment, Complex fabrication and operation
allows observation of cell migration
and invasion
Okazaki et al. (2020) [31] Gene editing Allows precise regulation of tumor-re- High technical requirements, potential
lated gene expression unpredictable side effects
Table 3. Comparison and application of 2D and 3D cell models and biological 3D printing methods in pancreatic cancer
Author Experimental Study Drug name Dosage Usage
method
Zhan et al. (2020) 2D cell culture Study A-2021-001: Testing the cytotoxicity of gem- Gemcitabine 10 μM Treatment for 24
[33] model citabine hydrochloride in 2D cell culture model hydrochloride for hours
using pancreatic cancer cell lines injection
Yu et al. (2021) [35] Animal model Study B-2022-002: Testing the in vivo anti-tumor Cisplatin 20 mg/kg Intravenous
efficacy of cisplatin using different tumor cell lines, injection to
including pancreatic cancer cell lines animals
Cui et al. (2012) 3D cell model Study C-2023-003: Testing the 3D anti-tumor Etoposide hydro- 5 μg/mL Added to 3D cell
[37]
efficacy of etoposide hydrochloride using 3D cell chloride model culture,
models of pancreatic cancer and other tumor types 72-hour treat-
ment
Szlasa et al. (2023) Integrated model Study A-2021-004: Testing the cytotoxicity of Gemcitabine 50 μM Added to culture
[36] gemcitabine hydrochloride in 2D model using hydrochloride for medium, 48-
pancreatic cancer cell lines injection hour treatment
Liang et al. (2017) Xenograft model Study D-2024-005: Testing the anti-tumor efficacy Albumin-bound 2 mg/kg Intraperitoneal
[38] of albumin-bound paclitaxel in xenograft model paclitaxel injection to mice
using pancreatic cancer cell lines and other tumor
types
Rahnama et al. Human embryo Study E-2025-006: Testing the anti-tumor efficacy Albumin-bound 1 μM Added to culture
(2022) [39] transplant of albumin-bound paclitaxel in human embryo paclitaxel medium, 72-
transplant model using pancreatic cancer cell lines hour treatment
and other tumor types
Thummuri et al. Tumor fragment Study F-2026-007: Testing the growth inhibition Chemotherapy 2 μg/mL Added to culture
(2022) [6] transplant of tumor fragments by chemotherapy drug F using drug F medium, 48-
pancreatic cancer cell lines and other tumor types hour treatment
(Continued...)
Volume 10 Issue 1 (2024) 134 https://doi.org/10.36922/ijb.1256