Page 127 - IJB-10-2
P. 127
International Journal of Bioprinting 3D bioprinting for corneal regeneration
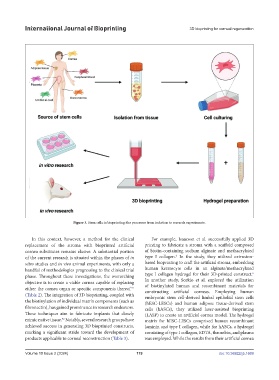
Figure 3. Stem cells in bioprinting: the processes from isolation to research experiments.
In this context, however, a method for the clinical For example, Isaacson et al. successfully applied 3D
replacement of the stroma with bioprinted artificial printing to fabricate a stroma with a scaffold composed
cornea substitutes remains elusive. A substantial portion of biotin-containing sodium alginate and methacrylated
6
of the current research is situated within the phases of in type I collagen. In the study, they utilized extrusion-
vitro studies and in vivo animal experiments, with only a based bioprinting to craft the artificial stroma, embedding
handful of methodologies progressing to the clinical trial human keratocyte cells in an alginate/methacrylated
6
phase. Throughout these investigations, the overarching type I collagen hydrogel for their 3D-printed construct.
objective is to create a viable cornea capable of replacing In another study, Sorkio et al. explored the utilization
either the cornea organ or specific components thereof of biotinylated human and recombinant materials for
91
constructing artificial corneas. Employing human
(Table 2). The integration of 3D bioprinting, coupled with embryonic stem cell-derived limbal epithelial stem cells
the biotinylation of individual matrix components (such as (hESC-LESCs) and human adipose tissue-derived stem
fibronectin), has gained prominence in research endeavors. cells (hASCs), they utilized laser-assisted bioprinting
These techniques aim to fabricate implants that closely (LASP) to create an artificial cornea model. The hydrogel
mimic native tissue. Notably, several research groups have matrix for hESC-LESCs comprised human recombinant
92
achieved success in generating 3D bioprinted constructs, laminin and type I collagen, while for hASCs, a hydrogel
marking a significant stride toward the development of consisting of type 1 collagen, EDTA, thrombin, and plasma
products applicable to corneal reconstruction (Table 3). was employed. While the results from their artificial cornea
Volume 10 Issue 2 (2024) 119 doi: 10.36922/ijb.1669