Page 45 - IJB-3-2
P. 45
Miaomiao Zhou, et. al.
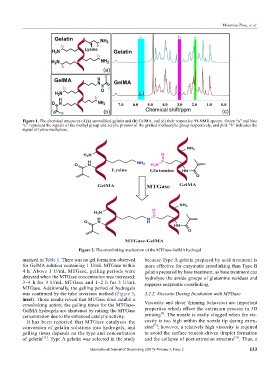
Figure 1. The chemical structures of (a) unmodified gelatin and (b) GelMA, and (c) their respective H-NMR spectra. Green “a” and blue
1
“c” represent the signals of the methyl group and acrylic protons of the grafted methacrylic group respectively, and pink “b” indicates the
signal of lysine methylene.
Figure 2. The crosslinking mechanism of the MTGase-GelMA hydrogel
marized in Table 1. There was no gel formation observed because Type A gelatin prepared by acid treatment is
for GelMA solution containing 1 U/mL MTGase within more effective for enzymatic crosslinking than Type B
4 h. Above 1 U/mL MTGase, gelling periods were gelatin prepared by base treatment, as base treatment can
detected when the MTGase concentration was increased: hydrolyse the amide groups of glutamine residues and
3–4 h for 3 U/mL MTGase and 1–2 h for 5 U/mL suppress enzymatic crosslinking.
MTGase. Additionally, the gelling period of hydrogels
was confirmed by the tube inversion method (Figure 3, 3.2.2. Viscosity During Incubation with MTGase
inset). Those results reveal that MTGase does exhibit a
crosslinking action; the gelling times for the MTGase- Viscosity and shear thinning behaviour are important
GelMA hydrogels are shortened by raising the MTGase properties which affect the extrusion process in 3D
[6]
concentration due to the enhanced catalytic activity. printing . The nozzle is easily clogged when the vis-
It has been reported that MTGase catalyses the cosity is too high within the nozzle tip during extru-
[35]
conversion of gelatin solutions into hydrogels, and sion ; however, a relatively high viscosity is required
gelling times depends on the type and concentration to avoid the surface tension-driven droplet formation
of gelatin [34] . Type A gelatin was selected in the study and the collapse of post-extrusion structure [36] . Thus, a
International Journal of Bioprinting (2017)–Volume 3, Issue 2 133