Page 112 - IJB-10-3
P. 112
International Journal of Bioprinting 3D bioprinting for vascularized skin tissue engineering
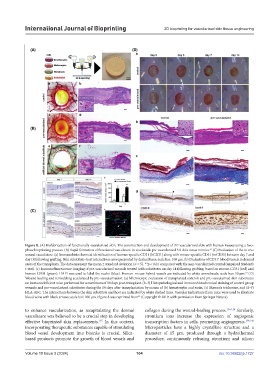
Figure 8. (A) Biofabrication of functionally vascularized skin. The construction and development of 3D vascularized skin with human tissues using a two-
63
phase bioprinting process. (B) Rapid formation of functional vasculature in vivo inside pre-vascularized 3D skin tissue mimics. (C) Evaluation of the in vivo
wound vasculature. (a) Immunohistochemical identification of human-specific CD31 (hCD31) along with mouse-specific CD31 (mCD31) between day 7 and
+
day 14 following grafting. Skin substitute–host interactions are represented by dashed lines; scale bar: 100 μm. (b) Evaluation of CD31 blood vessels in dermal
areas of the transplants. The data represent the mean ± standard deviation (n = 5). **p < 0.01 compared with the non-vascularized control (unpaired Student’s
t-test). (c) Immunofluorescence imaging of pre-vascularized wounds treated with substitutes on day 14 following grafting, based on mouse-CD31 (red) and
63
human-CD31 (green). DAPI was used to label the nuclei (blue). Human–mouse hybrid vessels are indicated by white arrowheads; scale bar: 50 μm. (D)
Wound healing and remodeling accelerated by pre-vascularization. (a) Microscopic evaluation of transplanted controls and pre-vascularized skin substitutes
on immunodeficient mice performed for a maximum of 14 days post-transplant. (b–f) Histopathological and immunohistochemical staining of control group
wounds and pre-vascularized substitutes during the 14 days after transplantation by means of (b) hematoxylin and eosin, (c) Masson’s trichrome, and (d–f)
HLA-ABC. The interactions between the skin substitute and host are indicated by white dashed lines. Vascular indications from mice were used to illustrate
63
blood veins with black arrows; scale bar: 100 μm. Figure 8 was reprinted from (Copyright © 2019, with permission from Springer Nature).
to enhance vascularization, as recapitulating the dermal collagen during the wound-healing process. 136-138 Similarly,
vasculature was believed to be a crucial step in developing strontium ions increase the expression of angiogenic
effective bioprinted skin replacements. In this context, transcription factors in cells, promoting angiogenesis. 139,140
135
incorporating therapeutic substances capable of stimulating Microparticles have a highly crystalline structure and a
blood vessel development into bioinks is crucial. Silica- diameter of 15 µm, produced through a hydrothermal
based products promote the growth of blood vessels and procedure, continuously releasing strontium and silicon
Volume 10 Issue 3 (2024) 104 doi: 10.36922/ijb.1727