Page 107 - IJB-10-3
P. 107
International Journal of Bioprinting 3D bioprinting for vascularized skin tissue engineering
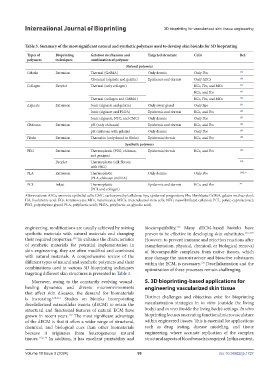
Table 3. Summary of the most significant natural and synthetic polymers used to develop skin bioinks for 3D bioprinting
Types of Bioprinting Gelation mechanism and Targeted structure Cells Ref.
polymers techniques combination of polymer
Natural polymers
Gelatin Extrusion Thermal (GelMA) Only dermis Only Fbs 179
Chemical (alginate and gelatin) Epidermis and dermis Only AECs 180
Collagen Droplet Thermal (only collagen) KCs, Fbs, and MCs 134
KCs, and Fbs 181
Thermal (collagen and GelMA) KCs, Fbs, and MCs 182
Alginate Extrusion Ionic (alginate and gelatin) Only sweat gland Only Eps 183
Ionic (alginate and PLGA) Epidermis and dermis KCs, and Fbs 184
Ionic (alginate, NFC, and CMC) Only dermis Only Fbs 185
Chitosan Extrusion pH (only chitosan) Epidermis and dermis KCs, and Fbs 186
pH (chitosan with gelatin) Only dermis Only Fbs 187
Fibrin Extrusion Thrombin (only found in fibrin) Epidermis/dermis KCs, and Fbs 188
Synthetic polymers
PEG Extrusion Thermoplastic (PEG, chitosan, Epidermis/dermis KCs, and Fbs 189
and genipin)
Droplet Thermoplastic (silk fibroin 190
with PEG)
PLA Extrusion Thermoplastic Only dermis Only Fbs 188,191
(PLA, chitosan and HA)
PCL Inkjet Thermoplastic Epidermis and dermis KCs, and Fbs 84
(PCL and collagen)
Abbreviations: AECs, amniotic epithelial cells; CMC, carboxymethyl cellulose; Eps, epidermal progenitors; Fbs, fibroblasts; GelMA, gelatin methacryloyl;
HA, hyaluronic acid; KCs, keratinocytes; MCs, melanocytes; MSCs, mesenchymal stem cells; NFC, nanofibrillated cellulose; PCL, poly(e-caprolactone);
PEG, polyethylene glycol; PLA, poly(lactic acid); PLGA, poly(lactic-co-glycolic acid).
115
engineering, modifications are usually achieved by mixing biocompatibility. Many dECM-based bioinks have
synthetic materials with natural materials and changing proven to be effective in developing skin substitutes. 116-119
their required properties. To enhance the characteristics However, to prevent immune and rejection reactions after
109
of synthetic materials for potential implementation in transplantation, physical, chemical, or biological removal
skin engineering, they are often modified and combined of biocompatible complexes from native tissues, which
with natural materials. A comprehensive review of the may damage the microstructure and bioactive substances
different types of natural and synthetic polymers and their within the ECM, is necessary. Decellularization and the
120
combinations used in various 3D bioprinting techniques optimization of these processes remain challenging.
targeting different skin structures is presented in Table 3.
Moreover, owing to the constantly evolving wound- 5. 3D bioprinting-based applications for
healing dynamics and diverse microenvironments engineering vascularized skin tissue
that affect skin diseases, the demand for biomaterials
is increasing. 110,111 Studies on bioinks incorporating Distinct challenges and objectives exist for bioprinting
decellularized extracellular matrix (dECM) to retain the vascularization strategies in in vitro (outside the living
structural and functional features of natural ECM have body) and in vivo (inside the living body) settings. In vitro
grown in recent years. The most significant advantage bioprinting focuses on creating functional microvasculature
112
of the dECM is that it offers a wider range of structural, within engineered tissues. This is essential for applications
chemical, and biological cues than other biomaterials such as drug testing, disease modeling, and tissue
because it originates from heterogeneous natural engineering, where accurate replication of the complex
tissues. 113,114 In addition, it has excellent printability and structural aspects of blood vessels is required. In this context,
Volume 10 Issue 3 (2024) 99 doi: 10.36922/ijb.1727