Page 521 - IJB-10-3
P. 521
International Journal of Bioprinting Drop-on-demand laser bioprinting
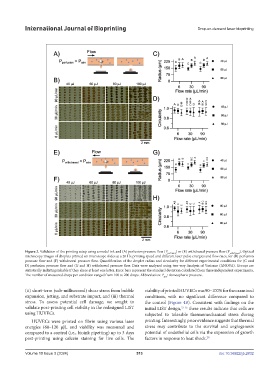
Figure 2. Validation of the printing setup using a model ink and (A) perfusion pressure flow (P perfusion ) or (E) withdrawal pressure flow (P withdrawal ). Optical
microscopy images of droplets printed on microscope slides at a 20 Hz printing speed and different laser pulse energies and flow rates, for (B) perfusion
pressure flow and (F) withdrawal pressure flow. Quantification of the droplet radius and circularity for different experimental conditions for (C and
D) perfusion pressure flow and (G and H) withdrawal pressure flow. Data were analyzed using two-way Analysis of Variance (ANOVA). Groups are
statistically indistinguishable if they share at least one letter. Error bars represent the standard deviation calculated from three independent experiments.
The number of measured drops per condition ranged from 100 to 200 drops. Abbreviation: P : Atmospheric pressure.
atm
(ii) short-term (sub-millisecond) shear stress from bubble viability of printed HUVECs was 90–100% for the examined
expansion, jetting, and substrate impact, and (iii) thermal conditions, with no significant difference compared to
stress. To assess potential cell damage, we sought to the control (Figure 4B). Consistent with findings on the
validate post-printing cell viability in the redesigned LIST initial LIST design, 23-25 these results indicate that cells are
using HUVECs. subjected to tolerable thermomechanical stress during
HUVECs were printed on fibrin using various laser printing. Interestingly, prior evidence suggests that thermal
energies (60–120 μJ), and viability was measured and stress may contribute to the survival and angiogenesis
compared to a control (i.e., bioink pipetting) up to 3 days potential of endothelial cells via the expression of growth
post-printing using calcein staining for live cells. The factors in response to heat shock.
28
Volume 10 Issue 3 (2024) 513 doi: 10.36922/ijb.2832