Page 114 - IJB-4-1
P. 114
Formation of cell spheroids using Standing Surface Acoustic Wave (SSAW)
acoustically formed cell spheroids and suspended HepG2 reduce the thermal effects on the viability of the formed
the control group (p = 0.492, 0.849, 0.566, and 0.492 on day cell spheroids. The temperature of PDMS cavity was
1, 3, 5, 7, respectively, all p> 0.05, see Figure 5D). Both measured to be around 26 C by an infrared thermometer
experimental and control group had high cell viability (MAX IR Thermometer, Fluke, Everett, WA USA).
over 90% which represents healthy cell condition and Nevertheless, the acoustic radiation force at the pressure
suggests the safety of our approach. It is found that the node for the generation of cell spheroid has a theoretical
cell viability by the high-frequency excitation was magnitude of 0. In this experiment, the cell spheroids in
slightly lower than that by the low-frequency excitation the diameter range of about 15 μm to 70 μm were over
despite without statistical difference (p< 0.05), which may
be due to greater acoustic radiation force applied to the 90% in viability after at least 7 days of cell culture. This
[36,66,67]
cells. The slight decrease of cell viability over time is due result is in good agreement with previous studies
to the cell spheroids being cultured in non-attachable where the cell spheroids in diameter below 100 μm could
environment. If transferred to a scaffold, cell spheroids survive at a very high percentage (over 85%). However,
will be able to grow into a stable construct. large cell spheroids may also result in some dead cells at
There are two major contributions to the death of cell the center after incubation for a long time. Such limitation
spheroids formed after acoustic manipulation: temperature of spheroid size is dependent on the type of cells and the
and magnitude of acoustic radiation force applied to the conditions of cell culture. As for hepatocyte, the mostly
cells during the acoustic excitation for approximately 30 viable spheroid diameter could reach about 120-180 μm [66–
min continuously. As the cell viability is highly sensitive 70] . Since oxygen is difficult to permeate through the
to the environment temperature, a lab-built cooling plate thick cell structure, further increase in size results in a
was placed underneath the LiNbO 3 substrate to release depletion of oxygen (hypoxic conditions) and causes cell
the excessive heat and control the temperature in order to necrosis in the core of large spheroids [65,71] .
(A) (B) (C)
(D)
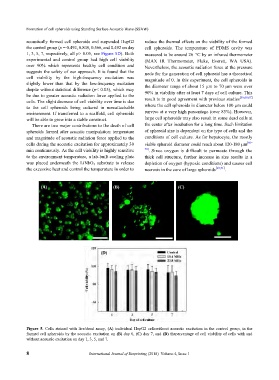
Figure 5. Cells stained with live/dead assay, (A) individual HepG2 cellswithout acoustic excitation in the control group, in the
formed cell spheroids by the acoustic excitation on (B) day 0, (C) day 7, and (D) thepercentage of cell viability of cells with and
without acoustic excitation on day 1, 3, 5, and 7.
8 International Journal of Bioprinting (2018)–Volume 4, Issue 1