Page 81 - IJB-4-1
P. 81
Coaxial nozzle-assisted electrohydrodynamic printing for microscale 3D cell-laden constructs
further verified that the printed alginate solution could nozzle-assisted electrohydrodynamic printing strategy
be instantly crosslinked to form hydrogel filament with could effectively fabricate the 3D cell-laden constructs
uniform dimension. Since the height of each layer was with high resolution, uniform cell distribution and high
close to the size of living cells, it might enable to print cell viability.
the filaments with single layer of cells in the vertical
direction for high-resolution cell printing. 4. Conclusion
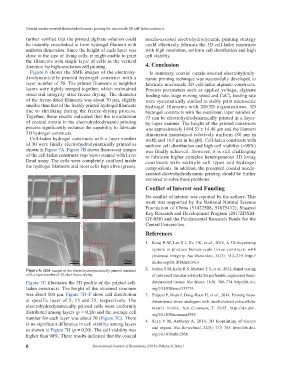
Figure 6 shows the SME images of the elec tro hy- In summary, coaxial nozzle-assisted elec tro hy dro dy-
dro dynamically printed hydrogel construct with a nam ic printing technique was successfully developed to
layer number of 50. The printed filaments at neighbor fabricate microscale 3D cell-laden alginate constructs.
layers were tightly merged together, which maintained Process parameters such as applied voltage, alginate
structural integrity after freeze drying. The diameter feeding rate, stage moving speed and CaCl feeding rate
2
of the freeze-dried filaments was about 70 μm, slightly were systematically studied to stably print microscale
smaller than that of the freshly printed hydrogel filaments hydrogel filaments with 2D/3D organizations. 3D
due to shrinking during the freeze-drying process. hydrogel constructs with the maximum layer number of
Together, these results indicated that the introduction 73 can be electrohydrodynamically printed in a layer-
of coaxial nozzle in the electrohydrodynamic printing by-layer manner. The height of the printed constructs
process significantly enhance the capability to fabricate was approximately 1464.53 ± 14.46 μm and the filament
3D hydrogel constructs. dimension maintained relatively uniform (80 μm in
Cell-laden hydrogel constructs with a layer number width and 18.5 μm in height). Cell-laden constructs with
of 30 were finally electrohydrodynamically printed as uniform cell distribution and high cell viability (>90%)
shown in Figure 7A. Figure 7B shows fluorescent images was finally achieved. However, it is still challenging
of the cell-laden constructs (top view) stained with Live/ to fabricate higher complex heterogeneous 3D living
Dead assay. The cells were completely confined inside constructs with multiple cell types and hydrogel
the hydrogel filaments and most cells kept alive (green). compositions. In addition, the presented coaxial nozzle-
assisted electrohydrodynamic printing should be further
explored to solve these problems.
Conflict of Interest and Funding
No conflict of interest was reported by the authors. This
work was supported by the National Natural Science
Foundation of China (51422508, 51675412), Shaanxi
Key Research and Development Program (2017ZDXM-
GY-058) and the Fundamental Research Funds for the
Central Universities.
References
1. Kang H-W, Lee S J, Ko I K, et al., 2016, A 3D bioprinting
system to produce human-scale tissue constructs with
structural integrity. Nat Biotechno, 34(3): 313–319. http://
dx.doi.org/10.1038/nbt.3413
2. Jordan S M, Kelly R S, Michael T Y, et al., 2012, Rapid casting
Figure 6. SEM images of the electrohydrodynamically printed construct
with a layer number of 50 after freeze drying of patterned vascular networks for perfusable engineered three-
Figure 7C illustrates the 3D profile of the printed cell- dimensional tissues. Nat Mater, 11(9): 768–774. http://dx.doi.
laden constructs. The height of the obtained structure org/10.1038/nmat335733
was about 500 μm. Figure 7D–F show cell distribution 3. Falguni P, Jinah J, Dong-Heon H, et al., 2014, Printing three-
at specific layer of 5, 15 and 25, respectively. The dimensional tissue analogues with decellularized extracellular
electrohydrodynamically printed cells were uniformly matrix bioink. Nat Commun, 5: 3935. http://dx.doi.
distributed among layers (p = 0.26) and the average cell org/10.1038/ncomms4935
number for each layer was about 70 (Figure 7G). There 4. Sean V M, Anthony A, 2014, 3D bioprinting of tissues
is no significant difference in cell viability among layers
as shown in Figure 7H (p = 0.20). The cell viability was and organs. Nat Biotechnol, 32(8): 773–785. http://dx.doi.
higher than 90%. These results indicated that the coaxial org/10.1038/nbt.2958
6 International Journal of Bioprinting (2018)–Volume 4, Issue 1