Page 543 - IJB-10-4
P. 543
International Journal of Bioprinting 3D-bioprinting of osteochondral plugs
quantity of growth medium and mixed with bioink. Thin with calcein AM/ethidium homodimer-III according to
disks of cell-laden chondral bioink containing 20 × 10 the manufacturer’s instructions (Biotium, USA). Samples
6
cells/mL were extruded into silicone molds (diameter: of bone bioink were collected at days 1 and 7 while chondral
6 mm; height: 1.6 mm), photocured for 60 s using a 405 bioink samples were collected at days 1, 7, 28, and 56 and
nm LED lamp, placed in growth media, and incubated imaged with a confocal microscope (STELLARIS 5; Leica
in a 37°C humidified cell culture incubator. After 24 h, Microsystems Inc., USA).
disks were placed in a differentiation medium. Chondral
disks were cultured in human mesenchymal stem cell 2.8. OsteoImage assay
(hMSC) chondrogenic differentiation medium (PT-3003; To visualize HAp deposits in the bone bioinks, gels
Lonza, USA) supplemented with 10 ng/mL TGF-β3 cultured in either osteogenic or growth media for 28 days
(PeproTech, USA) for up to 56 days. Bone disks (5 wt% were assessed using a mineralization assay (OsteoImage;
GelMA bioink containing 5 × 10 cells/mL) were cultured Lonza, USA) according to the manufacturer’s instructions
6
in either RoosterNourish-MSC growth medium or hMSC and imaged with an inverted fluorescence microscope
osteogenic differentiation medium (PT-3002; Lonza, USA) (Eclipse Ts2; Nikon Instruments Inc., USA) and a color
for up to 28 days. Media were changed three times per camera (CoolSnap DYNO; Photometrics, USA).
week. The material used to 3D print the PCL and ceramic 2.9. Histology and immunohistochemistry
reinforcing lattice of the subchondral bone section was Disks were fixed after 1 or 56 days of culture in neutral-
also evaluated for biocompatibility. 3D-printed disks were
seeded with 2 × 10 cells in 50 µL growth medium and buffered formalin overnight and then stored at 4°C in
5
allowed to attach for 2–3 h. Disks were cultured in 24-well phosphate-buffered saline (PBS). The hydrogel disks
plates with 1 mL growth media for up to 14 days. were then paraffin-embedded, sliced, and stained with
alcian blue (pH 1.0) or anti-collagen type II (COL II,
2.7. Live/dead assay mouse polyclonal anti-collagen type II; Abcam, USA) to
To assess cell viability, hydrogel disks were sliced in half to observe sulfated GAG and type II collagen. Brightfield
expose a rectangular cross-section of the gel and stained images of sections were taken using an automated slide
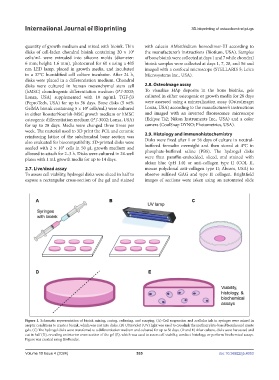
Figure 1. Schematic representation of bioink mixing, curing, culturing, and assaying. (A) Cell suspension and acellular ink in syringes were mixed in
aseptic conditions to create a bioink, which was cast into disks. (B) Ultraviolet (UV) light was used to crosslink the methacrylate-based bioinks and create
gels. (C) The hydrogel disks were transferred to a differentiation medium and cultured for up to 56 days. (D and E) After culture, disks were harvested and
cut in half (D), revealing an interior cross-section of the gel (E), which was used to assess cell viability, conduct histology, or perform biochemical assays.
Figure was created using BioRender.
Volume 10 Issue 4 (2024) 535 doi: 10.36922/ijb.4053