Page 546 - IJB-10-4
P. 546
International Journal of Bioprinting 3D-bioprinting of osteochondral plugs
−1
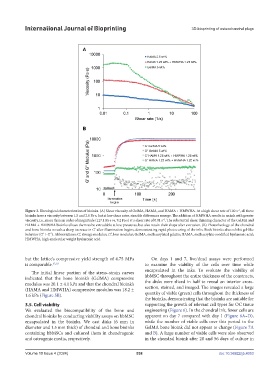
Figure 3. Rheological characterization of bioinks. (A) Shear viscosity of GelMA, HAMA, and HAMA + HMWHA. At a high shear rate of 100 s , all three
bioinks have a viscosity between 1.5 and 2.0 Pa·s, but at low shear rates, sizeable differences emerge. The addition of HMWHA results in an ink with greater
−1
viscosity, i.e., more than an order of magnitude (127.1 Pa·s vs. 9.2 Pa·s) at a shear rate of 0.01 s . The substantial shear thinning character of the GelMA and
HAMA + HMWHA bioinks allows them to be extrudable at low pressures, but also retain their shape after extrusion. (B) Photorheology of the chondral
and bone bioinks reveals a sharp increase in G’ after illumination begins, demonstrating rapid photocuring of the inks. Both bioinks also exhibit gel-like
behavior (G’ > G”). Abbreviations: G’, storage modulus; G”, loss modulus; GelMA, methacrylated gelatin; HAMA, methacrylate-modified hyaluronic acid;
HMWHA, high-molecular weight hyaluronic acid.
but the lattice’s compressive yield strength of 4.75 MPa On days 1 and 7, live/dead assays were performed
is comparable. 52,53 to examine the viability of the cells over time while
encapsulated in the inks. To evaluate the viability of
The initial linear portion of the stress–strain curves
indicated that the bone bioink’s (GelMA) compressive hbMSC throughout the entire thickness of the constructs,
modulus was 20.1 ± 4.1 kPa and that the chondral bioink’s the disks were sliced in half to reveal an interior cross-
(HAMA and HMWHA) compressive modulus was 18.2 ± section, stained, and imaged. The images revealed a large
1.6 kPa (Figure 5B). quantity of viable (green) cells throughout the thickness of
the bioinks, demonstrating that the bioinks are suitable for
3.5. Cell viability supporting the growth of relevant cell types for OC tissue
We evaluated the biocompatibility of the bone and engineering (Figure 6). In the chondral ink, fewer cells are
chondral bioinks by conducting viability assays on hbMSC apparent on day 7 compared with day 1 (Figure 6A–D),
encapsulated in the bioinks. We cast disks (6 mm in while the number of viable cells over this period in the
diameter and 1.5 mm thick) of chondral and bone bioinks GelMA bone bioink did not appear to change (Figure 7A
containing hbMSCs and cultured them in chondrogenic and B). A large number of viable cells were also observed
and osteogenic media, respectively. in the chondral bioink after 28 and 56 days of culture in
Volume 10 Issue 4 (2024) 538 doi: 10.36922/ijb.4053