Page 551 - IJB-10-4
P. 551
International Journal of Bioprinting 3D-bioprinting of osteochondral plugs
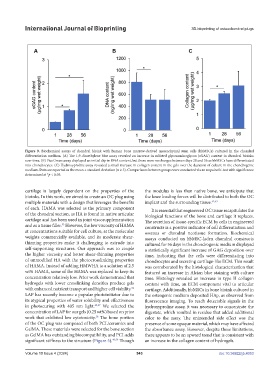
Figure 9. Biochemical assays of chondral bioink with human bone marrow-derived mesenchymal stem cells (hbMSCs) cultured in the chondral
differentiation medium. (A) The 1,9-dimethylene blue assay revealed an increase in sulfated glycosaminoglycan (sGAG) content in chondral bioinks
over time. (B) PicoGreen assay displayed an initial dip in DNA content, but there were no changes between days 28 and 56 as hbMSCs have differentiated
into chondrocytes. (C) Hydroxyproline assay revealed a small increase in collagen content in the gels over the duration of culture in the chondrogenic
medium. Data are reported as the mean ± standard deviation (n ≥ 3). Comparisons between groups were conducted via an unpaired t-test with significance
determined at *p < 0.05.
cartilage is largely dependent on the properties of the the modulus is less than native bone, we anticipate that
bioinks. In this work, we aimed to create an OC plug using the knee loading forces will be distributed to both the OC
multiple materials with a design that leverages the benefits implant and the surrounding tissue. 52,53
of each. HAMA was selected as the primary component It is essential that engineered OC tissue recapitulates the
of the chondral section, as HA is found in native articular biological functions of the bone and cartilage it replaces.
cartilage and has been used in joint viscosupplementation The secretion of tissue-specific ECM by cells in engineered
and as a tissue filler. However, the low viscosity of HAMA constructs is a positive indicator of cell differentiation and
55
at concentrations suitable for cell culture, at the molecular osseous or chondral neotissue formation. Biochemical
weights commercially available, and its moderate shear- assays conducted on hbMSC-laden chondral constructs
thinning properties make it challenging to extrude into cultured for 56 days in the chondrogenic medium displayed
self-supporting structures. Our approach was to couple a statistically significant increase of GAG deposition over
the higher viscosity and better shear-thinning properties time, indicating that the cells were differentiating into
of unmodified HA with the photocrosslinking properties chondrocytes and secreting cartilage-like ECM. This result
of HAMA. Instead of adding HMWHA to a solution of 2.5 was corroborated by the histological characterization that
wt% HAMA, some of the HAMA was replaced to keep its featured an increase in Alcian blue staining with culture
concentration relatively low. Prior work demonstrated that time. Histology revealed an increase in type II collagen
hydrogels with lower crosslinking densities produce gels content with time, an ECM component vital to articular
with enhanced nutrient transport and higher cell viability. cartilage. Additionally, hbMSCs in bone bioink cultured in
56
LAP has recently become a popular photoinitiator due to the osteogenic medium deposited HAp, as observed from
its atypical properties of water solubility and effectiveness fluorescence imaging. To reach detectable signals in the
in photocuring with 405 nm light. 49,57 We selected the hydroxyproline assay, it was necessary to concentrate the
concentration of LAP for our gels (0.25 wt%) based on prior digestate, which resulted in residue that added additional
work that exhibited low cytotoxicity. The bone portion color to the assay. The unintended side effect was the
58
of the OC plug was composed of both PCL/ceramics and presence of some opaque material, which may have affected
GelMA. These materials were selected for the bone section the absorbance assay. However, despite these limitations,
as GelMA has outstanding biocompatibility, and PCL adds there appears to be an upward trend that is consistent with
significant stiffness to the structure (Figure 5). 49,59 Though an increase in the collagen content of hydrogels.
Volume 10 Issue 4 (2024) 543 doi: 10.36922/ijb.4053