Page 18 - IJB-5-2
P. 18
Development and characterisation of a photocurable alginate bioink for 3D bioprinting
Where τ is the shear stress (Pa), is the shear rate (s ), 2.4 Characterization of Photocrosslinked
-1
ɳ the consistency index or equivalent viscosity (Pa. s), Hydrogels
and n is the power law index (dimensionless) that varies
as follows: 2.4.1 Morphological characterization
n<1: Shear-thinning system; The morphology of the internal structure of the hydrogels
n=1: Newtonian system; was investigated through scanning electron microscopy
n>1: Shear-thickening system. (SEM), using the Hitachi S3000N VPSEM system.
Alginate samples were produced using cylindrical
2.3 Hydrogel Formation molds (8 mm diameter and 4 mm high). After hydrogel
Photocrosslinked alginate methacrylate hydrogels formation, samples were extensively washed in diH O,
2
were prepared by dissolving 2% w/v alginate frozen at −80°C and lyophilized. Samples were fixed on
methacrylate with different photoinitiator concentration stubs using double-sided adhesive tape and sputter coated
solutions (0.5-1.5% w/v) of VA-086 photoinitiator with platinum sputter-coating.
solutions (2,2’-Azobis[2-methyl-N-(2-hydroxyethyl) 2.4.2 Mechanical characterization
propionamide azo initiator (Wako Pure Chemical
Industries, USA). Cross-linked disks were produced by Compression tests were performed at a constant strain
pipetting the alginate solution in an acrylic mold with rate using the Instron 3344 machine equipped with
8 mm diameter and 4 mm height. Photopolymerization a 10-N load cell (Instron, Buckinghamshire, UK).
Cross-linked alginate hydrogel disks were prepared
was conducted using a 365 nm UV light (Model as described in section (2.2) and maintained in diH O
Dymax 2000-EC, Dymax Europe GmbH, Wiesbaden, at 37ºC following the protocol described by Jeon
2
Germany) irradiating at 8 mW/cm during 8 min. et al. [27] . After 24 h of incubation, swollen alginate
2
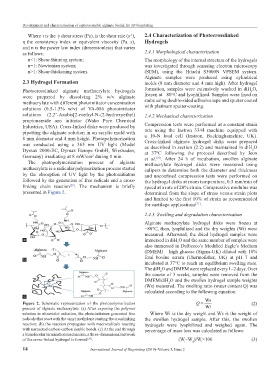
The photopolymerization process of alginate methacrylate hydrogel disks were measured using
methacrylate is a radicular polymerization process started calipers to determine both the diameter and thickness
by the absorption of UV light by the photoinitiators and unconfined compression tests were performed on
followed by the generation of free radicals and a cross- the hydrogel disks at room temperature, 0.5 mm/min of
linking chain reaction . The mechanism is briefly speed at a rate of 20% strain. Compressive modulus was
[29]
presented in Figure 2. determined from the slope of stress versus strain plots
and limited to the first 10% of strain as recommended
for cartilage applications [31] .
2.4.3. Swelling and degradation characterization
Alginate methacrylate hydrogel disks were frozen at
−80°C, then, lyophilized and the dry weights (Wi) were
measured. Afterward, the dried hydrogel samples were
a immersed in diH O and the same number of samples were
2
also immersed in Dulbecco’s Modified Eagle’s Medium
(DMEM) – high glucose (Sigma-UK) diluted with 10%
fetal bovine serum (Thermofisher, UK) at pH 7 and
b
incubated at 37°C to reach an equilibrium swelling state.
The diH O and DMEM were replaced every 1–2 days. Over
2
the course of 3 weeks, samples were removed from the
DMEM/diH O and the swollen hydrogel sample weights
2
(Ws) measured. The swelling ratio (water content Q) was
calculated according to the following equation:
c
Figure 2. Schematic representation of the photopolymerization Q = Ws (2)
process of alginate methacrylate. (a) After exposing the polymer Wi
solution to ultraviolet radiation, the photoinitiators generated free Where Wi is the dry weight and Ws is the weight of
radicals that react with the vinyl methylene starting the crosslinking the swollen hydrogel sample. After this, the swollen
reaction; (b) the reaction propagates with macroradicals reacting hydrogels were lyophilized and weighed again. The
with unreacted carbon-carbon double bonds. (c) At the end through percentage of mass loss was calculated as follows:
a bimolecular termination mechanism, a three-dimensional network
of the cross-linked hydrogel is formed . (W–W )/W×100 (3)
[30]
i
i
d
14 International Journal of Bioprinting (2019)–Volume 5, Issue 2