Page 354 - IJB-10-5
P. 354
International Journal of Bioprinting Printing organoids in peptide matrices
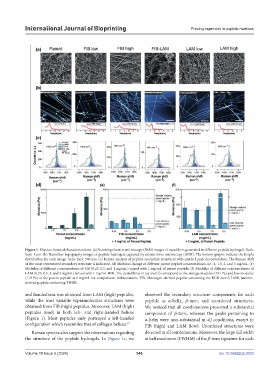
Figure 1. Physicochemical characterizations. (a) Scanning electron microscope (SEM) images of nanofibers generated in different peptide hydrogels. Scale
bars: 1 µm. (b) Nanofiber topography images of peptide hydrogels captured by atomic force microscopy (AFM). The bottom graphs indicate the height
distribution for each image. Scale bars: 500 nm. (c) Raman analysis of peptide secondary structures with amide I peak deconvolution. The Raman shift
of the most represented secondary structure is indicated. (d) Modulus change at different parent peptide concentrations, i.e., 1, 1.5, 2, and 3 mg/mL. (e)
Modulus of different concentrations of FIB (0.25, 0.5, and 1 mg/mL) mixed with 1 mg/mL of parent peptide. (f) Modulus of different concentrations of
LAM (0.25, 0.5, 1, and 2 mg/mL) mixed with 1 mg/mL IIFK. The dotted lines in (e) and (f) correspond to the storage modulus (731 Pa) and loss modulus
(119 Pa) of the parent peptide at 1 mg/mL for comparison. Abbreviations: FIB, fibrinogen-derived peptide containing the RGD motif; LAM, laminin-
derived peptide containing YIGSR.
and handedness was obtained from LAM (high) peptides, observed the secondary structure components for each
while the least variable supramolecular structures were peptide as α-helix, β-turn, and unordered structures.
obtained from FIB (high) peptides. Moreover, LAM (high) We noticed that all combinations presented a substantial
peptides result in both left- and right-handed helices component of β-turn, whereas the peaks pertaining to
(Figure 1). Most peptides only portrayed a left-handed α-helix were non-substantial in all conditions, except in
configuration which resembles that of collagen helices. FIB (high) and LAM (low). Unordered structures were
47
Raman spectra also support the observations regarding detected in all combinations. Moreover, the large full width
the structure of the peptide hydrogels. In Figure 1c, we at half maximum (FWHM) of the β-turn signature for each
Volume 10 Issue 5 (2024) 346 doi: 10.36922/ijb.3033