Page 505 - IJB-10-5
P. 505
International Journal of Bioprinting 3D bioprinting of full-thickness skin with a rete ridge structure
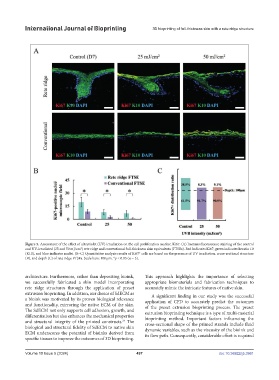
Figure 5. Assessment of the effect of ultraviolet (UV) irradiation on the cell proliferation marker, Ki67. (A) Immunofluorescence staining of the control
and UV-irradiated (25 and 50 mJ/cm ) rete ridge and conventional full-thickness skin equivalents (FTSEs). Red indicates Ki67, green indicates keratin 10
2
(K10), and blue indicates nuclei. (B–C) Quantitative analysis results of Ki67 cells are based on the presence of UV irradiation, cross-sectional structure
+
(B), and depth (C) of rete ridge FTSEs. Scale bars: 100 μm. *p < 0.05 (n = 3).
architecture. Furthermore, rather than depositing bioink, This approach highlights the importance of selecting
we successfully fabricated a skin model incorporating appropriate biomaterials and fabrication techniques to
rete ridge structures through the application of preset accurately mimic the intricate features of native skin.
extrusion bioprinting. In addition, our choice of SdECM as A significant finding in our study was the successful
a bioink was motivated by its proven biological relevance application of CFD to accurately predict the outcomes
and functionality, mirroring the native ECM of the skin. of the preset extrusion bioprinting process. The preset
The SdECM not only supports cell adhesion, growth, and extrusion bioprinting technique is a type of multi-material
differentiation but also enhances the mechanical properties bioprinting method. Important factors influencing the
and structural integrity of the printed constructs. The cross-sectional shape of the printed strands include fluid
24
biological and structural fidelity of SdECM to native skin dynamic variables, such as the viscosity of the bioink and
ECM underscores the potential of bioinks derived from its flow path. Consequently, considerable effort is required
specific tissues to improve the outcomes of 3D bioprinting.
Volume 10 Issue 5 (2024) 497 doi: 10.36922/ijb.3961