Page 58 - IJB-10-5
P. 58
International Journal of Bioprinting Medical regenerative in situ bioprinting
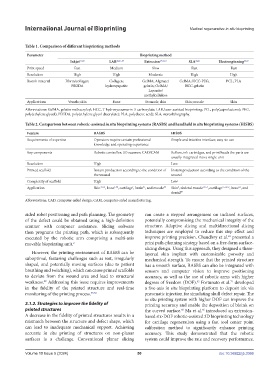
Table 1. Comparison of different bioprinting methods
Parameter Bioprinting method
Inkjet 37,48 LAB 40,41,49 Extrusion 29,50,51 SLA 44,45 Electrospinning 46,47
Print speed Fast Medium Slow Fast Fast
Resolution High High Moderate High High
Bioink material Fibrin/collagen; Collagen; GelMA; Alginate/ GelMA; HCC-PEG; PCL; PLA
PEGDA hydroxyapatite gelatin; GelMA/ HCC-gelatin
Laponite/
methylcellulose
Applications Vessels; skin Bone Stomach; skin Skin; muscle Skin
Abbreviations: GelMA, gelatin methacryloyl; HCC, 7-hydroxycoumarin-3-carboxylate; LAB, laser-assisted bioprinting; PCL, poly(caprolactone); PEG,
poly(ethylene glycol); PEGDA, poly(ethylene glycol diacrylate); PLA, poly(lactic acid); SLA, stereolithography.
Table 2. Comparison between robotic-assisted in situ bioprinting systems (RASBS) and handheld in situ bioprinting systems (HISBS)
Feature RASBS HISBS
Requirements of expertise Operators require certain professional Simple and intuitive interface; easy-to-use
knowledge and operating experience
Key components Robotic controller; 3D scanner; CAD/CAM Rollers, ink cartridges, and print heads the parts are
usually integrated into a single unit
Resolution High Low
Printed scaffold Instant production according to the condition of Instant production according to the condition of the
the wound wound
Complexity of scaffold High Low
45
9
58
1
Application Skin 37,52 , bone , cartilage , brain , and muscle 44 Skin , skeletal muscle 53,54 , cartilage 55,56,57 , bone , and
1,40
dental 59
Abbreviations: CAD, computer-aided design; CAM, computer-aided manufacturing.
aided robot positioning and path planning. The geometry can create a stepped arrangement on inclined surfaces,
of the defect could be obtained using a high-definition potentially compromising the mechanical integrity of the
scanner with computer assistance. Slicing software structure. Adaptive slicing and multidirectional slicing
then programs the printing path, which is subsequently techniques are employed to reduce this step effect and
62
executed by the robotic arm comprising a multi-axis improve printing precision. Chaudhry et al. presented a
movable bioprinting unit. 2 print path-planning strategy based on a free-form surface-
slicing design. Using this approach, they designed a three-
However, the printing environment of RASBS can be layered skin implant with customizable porosity and
suboptimal, featuring challenges such as wet, irregularly mechanical strength. To ensure that the printed structure
shaped, and potentially moving surfaces (due to patient has a smooth surface, RASBS can also be integrated with
breathing and twitching), which can cause printed scaffolds sensors and computer vision to improve positioning
to deviate from the wound area and lead to structural accuracy, as well as the use of robotic arms with higher
weakness. Addressing this issue requires improvements degrees of freedom (DOF). Fortunato et al. developed
62
71
28
in the fidelity of the printed structure and real-time a five-axis in situ bioprinting platform to deposit ink via
monitoring of the printing process. 35,50 pneumatic injection for simulating skull defect repair. The
in situ printing system with higher DOF can improve the
2.1.2. Strategies to improve the fidelity of printing accuracy and enable the deposition of bioink on
printed structures the curved surface. Ma et al. introduced an extrusion-
68
66
A decrease in the fidelity of printed structures results in a based six-DOF robotic-assisted 3D bioprinting technology
mismatch between the structure and defect shape, which for cartilage regeneration using a fast tool center point
can lead to inadequate mechanical support. Achieving calibration method to significantly enhance printing
accurate in situ printing of structures on non-planar accuracy. This study demonstrated that the robotic
surfaces is a challenge. Conventional planar slicing system could improve the rate and recovery performance.
Volume 10 Issue 5 (2024) 50 doi: 10.36922/ijb.3366