Page 467 - IJB-9-2
P. 467
International Journal of Bioprinting Bioprinting of exosomes
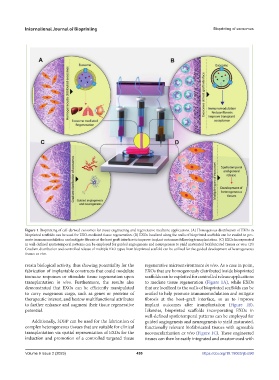
Figure 1. Bioprinting of cell-derived exosomes for tissue engineering and regenerative medicine applications. (A) Homogenous distribution of EXOs in
bioprinted scaffolds can be used for EXO-mediated tissue regeneration. (B) EXOs localized along the walls of bioprinted scaffolds can be availed to pro-
mote immunomodulation and mitigate fibrosis at the host graft interface to improve implant outcomes following transplantation. (C) EXOs incorporated
in well-defined spatiotemporal patterns can be employed for guided angiogenesis and neurogenesis to yield maturated biofabricated tissues ex vivo. (D)
Gradient distribution and controlled release of multiple EXO types from bioprinted scaffold can be utilized for the guided development of heterogeneous
tissues ex vivo.
retain biological activity, thus showing potentiality for the regenerative microenvironment in vivo. As a case in point,
fabrication of implantable constructs that could modulate EXOs that are homogenously distributed inside bioprinted
immune responses or stimulate tissue regeneration upon scaffolds can be exploited for controlled release applications
transplantation in vivo. Furthermore, the results also to mediate tissue regeneration (Figure 1A), while EXOs
demonstrated that EXOs can be efficiently manipulated that are localized to the walls of bioprinted scaffolds can be
to carry exogenous cargo, such as genes or proteins of availed to help promote immunomodulation and mitigate
therapeutic interest, and bestow multifunctional attributes fibrosis at the host–graft interface, so as to improve
to further enhance and augment their tissue regenerative implant outcomes after transplantation (Figure 1B).
potential. Likewise, bioprinted scaffolds incorporating EXOs in
well-defined spatiotemporal patterns can be employed for
Additionally, 3DBP can be used for the fabrication of guided angiogenesis and neurogenesis to yield maturated,
complex heterogeneous tissues that are suitable for clinical functionally relevant biofabricated tissues with agreeable
transplantation via spatial representation of EXOs for the neovascularization ex vivo (Figure 1C). These engineered
induction and promotion of a controlled targeted tissue tissues can then be easily integrated and anastomosed with
Volume 9 Issue 2 (2023) 459 https://doi.org/10.18063/ijb.690