Page 101 - IJB-9-5
P. 101
International Journal of Bioprinting Application and prospects of 3D printable microgels
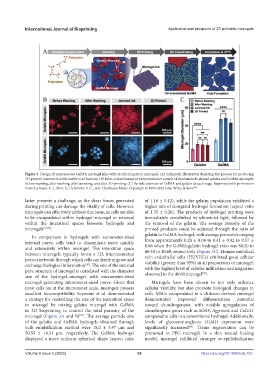
Figure 3. Design of microporous GelMA microgel inks with sacrificial gelatin microgels. (A) Schematic illustration depicting the process for producing
3D-printed constructs with tunable void fraction. (B) False-colored image of a representative sample of rhodamine B-stained gelatin and GelMA microgels
before washing, after washing, after jamming, and after 3D printing. (C) Particle diameter of GelMA and gelatin at each stage. Reprinted with permission
from Seymour, A. J., Shin, S., Heilshorn, S. C., Adv. Healthcare Mater. Copyright © 1999-2023 John Wiley & Sons .
[89]
latter presents a challenge, as the shear forces generated of 1.16 ± 0.12), while the gelatin population exhibited a
during printing can damage the vitality of cells. However, higher rate of elongated hydrogel formation (aspect ratio
microgels can effectively address this issue, as cells are able of 1.35 ± 0.26). The products of hydrogel printing were
to be encapsulated within hydrogel–microgel or retained immediately crosslinked by ultraviolet light, followed by
within the interstitial spaces between hydrogels and the removal of the gelatin. The average porosity of the
microgels [87,88] . printed products could be adjusted through the ratio of
In comparison to hydrogels with nanometer-sized gelatin to GelMA hydrogel, with average porosities ranging
internal pores, cells tend to disseminate more quickly from approximately 0.28 ± 0.04 to 0.41 ± 0.02 to 0.57 ±
and extensively within microgel. The interstitial space 0.06 when the GelMA:gelatin hydrogel ratio was 80:20 to
between microgels typically forms a 3D, interconnected 60:40 to 40:60, respectively (Figure 3C). Human umbilical
porous network through which cells can freely migrate and vein endothelial cells (HUVECs) exhibited good cellular
exchange biological information . The size of the internal viability (greater than 95%) in all proportions of microgel,
[88]
pore structure of microgel is correlated with the diameter with the highest level of cellular infiltration and migration
[89]
size of the hydrogel–microgel, with micrometer-sized observed in the 40:60 microgel .
microgel generating micrometer-sized pores. Given that Microgels have been shown to not only enhance
most cells are at the micrometer scale, microgels possess cellular viability, but also promote biological changes in
excellent biocompatibility. Seymour et al. demonstrated cells. MSCs encapsulated in a chitosan-derived microgel
a strategy for controlling the size of the interstitial space demonstrated improved differentiation potential
in microgel by mixing gelatin microgel with GelMA toward chondrogenesis, with notable upregulation of
in 3D bioprinting to control the total porosity of the chondrogenic genes such as SOX9, Aggrecan, and Col2A1
microgel (Figure 3A and B) . The average particle size compared to cells in a conventional hydrogel. Additionally,
[89]
of the gelatin and GelMA hydrogels obtained through levels of glycosaminoglycan (GAG) expression were
bulk emulsification method were 18.0 ± 3.97 µm and significantly increased . Tissue regeneration can be
[68]
50.55 ± 14.31 µm, respectively. The GelMA hydrogel promoted in PEG microgel. In a skin wound healing
displayed a more uniform spherical shape (aspect ratio model, microgel exhibited stronger re-epithelialization
Volume 9 Issue 5 (2023) 93 https://doi.org/10.18063/ijb.753