Page 57 - v11i4
P. 57
International Journal of Bioprinting 3D bioprinting of nerve guidance conduits
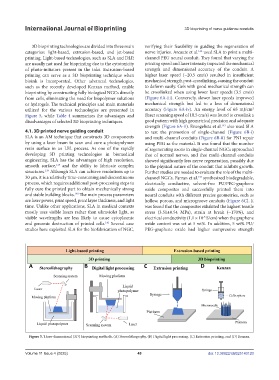
3D bioprinting technologies are divided into three main verifying their feasibility in guiding the regeneration of
categories: light-based, extrusion-based, and jet-based nerve injuries. Arcaute et al. used SLA to print a multi-
142
printing. Light-based technologies, such as SLA and DLP, channel PEG neural conduit. They found that varying the
are usually not used for bioprinting due to the cytotoxicity printing speed and laser intensity impacted the mechanical
of photo-initiators present in the inks. Extrusion-based strength and dimensional accuracy of the conduit. A
printing can serve as a 3D bioprinting technique when higher laser speed (~20.5 cm/s) resulted in insufficient
bioink is incorporated. Other advanced technologies, mechanical strength post-crosslinking, causing the conduit
such as the recently developed Kenzan method, enable to deform easily. Gels with good mechanical strength can
bioprinting by constructing fully biological NGCs directly be crosslinked when using lower laser speeds (3.5 cm/s)
from cells, eliminating the need for biopolymer solutions (Figure 6A-iii). Conversely, slower laser speeds improved
or hydrogels. The technical principles and main materials mechanical strength but led to a loss of dimensional
2
utilized for the various technologies are presented in accuracy (Figure 6A-iv). An energy level of 65 mJ/cm
Figure 5, while Table 4 summarizes the advantages and (laser scanning speed of 10.5 cm/s) was found to crosslink a
disadvantages of selected 3D bioprinting techniques. good pattern with high geometrical precision and adequate
strength (Figure 6A-ii). Evangelista et al. also used SLA
143
4.1. 3D printed nerve guiding conduit to test the promotion of single-channel (Figure 6B-i)
SLA is an AM technique that constructs 3D components and multi-channel conduits (Figure 6B-ii) for PNI repair
by using a laser beam to scan and cure a photopolymer using PEG as the material. It was found that the number
resin surface in an LBL process. As one of the rapidly of regenerating axons in single-channel NGCs approached
developing 3D printing technologies in biomedical that of normal nerves, and that multi-channel conduits
engineering, SLA has the advantages of high resolution, showed significantly less nerve regeneration, possibly due
138
smooth surface, and the ability to fabricate complex to the physical nature of the conduit that inhibits growth.
structures. Although SLA can achieve resolutions up to Further studies are needed to evaluate the role of the multi-
139
30 μm, it is a relatively time-consuming and discontinuous channel NGCs. Farzan et al. synthesized biodegradable,
144
process, which requires additional post-processing steps to electrically conductive, solvent-free PU/PEG-graphene
fully cure the printed part to obtain mechanically strong oxide composites and successfully printed them into
and stable building blocks. The main process parameters neural conduits with different precise geometries, such as
140
are laser power, print speed, print layer thickness, and light hollow, porous, and microgroove conduits (Figure 6C). It
time. Unlike other applications, SLA in medical contexts was found that the composites exhibited the highest tensile
mostly uses visible lasers rather than ultraviolet light, as stress (3.51±0.54 MPa), strain at break (~170%), and
visible wavelengths are less likely to cause cytoplasmic electrical conductivity (1.1 × 10 S/cm) when the graphene
-3
and genomic destruction of printed cells. Several case oxide content was set at 5 wt%. In addition, 5 wt% PU/
141
studies have exploited SLA for the biofabrication of NGC, PEG-graphene oxide had higher compressive strength
Figure 5. Three-dimensional (3D) bioprinting methods. (A) Stereolithography, (B) Digital light processing, (C) Extrusion printing, and (D) Kenzan.
Volume 11 Issue 4 (2025) 49 doi: 10.36922/IJB025140120