Page 44 - ITPS-6-1
P. 44
38 INNOSC Theranostics and Pharmacological Sciences, 2023, Vol. 6, No. 1 Hariharan
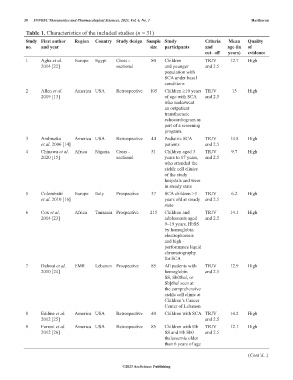
Table 1. Characteristics of the included studies (n = 31)
Study First author Region Country Study design Sample Study Criteria Mean Quality
no. and year size participants and age (in of
cut ‑ off years) evidence
1 Agha et al. Europe Egypt Cross - 80 Children TRJV 12.7 High
2014 [22] sectional and younger and 2.5
population with
SCA under basal
conditions
2 Allen et al. America USA Retrospective 105 Children ≥10 years TRJV 15 High
2019 [13] of age with SCA and 2.5
who underwent
an outpatient
transthoracic
echocardiogram as
part of a screening
program.
3 Ambrusko America USA Retrospective 44 Pediatric SCA TRJV 14.8 High
et al. 2006 [14] patients and 2.5
4 Chinawa et al. Africa Nigeria Cross - 51 Children aged 3 TRJV 9.7 High
2020 [15] sectional years to 17 years, and 2.5
who attended the
sickle cell clinics
of the study
hospitals and were
in steady state
5 Colombatti Europe Italy Prospective 37 SCA children >3 TRJV 6.2 High
et al. 2010 [16] years old at steady and 2.5
state
6 Cox et al. Africa Tanzania Prospective 215 Children and TRJV 14.1 High
2014 [23] adolescents aged and 2.5
9–19 years, HbSS
by hemoglobin
electrophoresis
and high -
performance liquid
chromatography
for SCA
7 Dahoui et al. EMR Lebanon Prospective 85 All patients with TRJV 12.9 High
2010 [24] hemoglobin and 2.5
SS, Sb0thal, or
Sbþthal seen at
the comprehensive
sickle cell clinic at
Children’s Cancer
Center of Lebanon
8 Eddine et al. America USA Retrospective 40 Children with SCA TRJV 14.2 High
2012 [25] and 2.5
9 Forrest et al. America USA Retrospective 85 Children with Hb TRJV 12.1 High
2012 [26] SS and Hb Sb0 and 2.5
thalassemia older
than 6 years of age
(Cont’d...)
©2023 AccScience Publishing