Page 54 - ITPS-8-2
P. 54
INNOSC Theranostics and
Pharmacological Sciences Management of heart failure in Pakistan
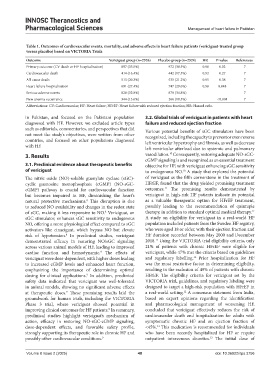
Table 1. Outcomes of cardiovascular events, mortality, and adverse effects in heart failure patients (vericiguat‑treated group
versus placebo) based on VICTORIA Trials
Outcome Vericiguat group (n=2526) Placebo group (n=2524) HR P‑value References
Primary outcome (CV death or HF hospitalization) 897 (35.5%) 972 (38.5%) 0.90 0.02 7
Cardiovascular death 414 (16.4%) 442 (17.5%) 0.92 0.27 7
All-cause death 513 (20.3%) 535 (21.2%) 0.95 0.38 7
Heart failure hospitalization 691 (27.4%) 747 (29.6%) 0.90 0.048 7
Serious adverse events 828 (32.8%) 878 (34.8%) - - 7
New anemia occurrence 344 (13.6%) 266 (10.5%) - <0.001 7
Abbreviations: CV: Cardiovascular; HF: Heart failure; HFrEF: Heart failure with reduced ejection fraction; HR: Hazard ratio.
in Pakistan, and focused on the Pakistani population 3.2. Global trials of vericiguat in patients with heart
diagnosed with HF. However, we excluded article types failure and reduced ejection fraction
such as editorials, commentaries, and perspectives that did Various potential benefits of sGC stimulators have been
not meet the study’s objectives, were written from other recognized, including the capacity to prevent or even reverse
countries, and focused on other populations diagnosed left ventricular hypertrophy and fibrosis, as well as decrease
with HF.
left ventricular afterload due to systemic and pulmonary
10
3. Results vasodilation. Consequently, restoring adequate NO-sGC-
cGMP signaling is and recognized as an essential treatment
3.1. Preclinical evidence about therapeutic benefits objective for HF, with vericiguat enhancing sGC sensitivity
of vericiguat to endogenous NO. A study that explored the potential
10
The nitric oxide (NO)-soluble guanylate cyclase (sGC)- of vericiguat as the fifth cornerstone in the treatment of
cyclic guanosine monophosphate (cGMP) (NO-sGC- HFrEF, found that the drug yielded promising treatment
11
cGMP) pathway is crucial for cardiovascular function outcomes. The promising results demonstrated by
but becomes impaired in HF, diminishing the heart’s vericiguat in high-risk HF patients indicate its potential
natural protective mechanisms. This disruption is due as a valuable therapeutic option for HFrEF treatment,
8
to reduced NO availability and changes in the redox state possibly leading to the recommendation of quintuple
11
of sGC, making it less responsive to NO. Vericiguat, an therapy, in addition to standard optimal medical therapy.
8
sGC stimulator, enhances sGC sensitivity to endogenous A study on eligibility for vericiguat in a real-world HF
NO, offering a more physiological effect compared to sGC population included patients from the Sweden HF registry,
activators like cinaciguat, which bypass NO but elevate who were aged 18 or older, with their ejection fraction and
risk of hypotension. In preclinical studies, vericiguat HF duration recorded between May 2000 and December
8
demonstrated efficacy in restoring NO/sGC signaling 2018. Using the VICTORIA trial eligibility criteria, only
12
across various animal models of HF, leading to improved 21% of patients with chronic HFrEF were eligible for
cardiac function and hemodynamic. The effects of vericiguat, while 47% met the criteria based on guidelines
9
12
vericiguat were dose-dependent, with higher doses leading and regulatory labelling. Prior hospitalization for HF
to increased cGMP levels and enhanced heart function, was the most restrictive factor in determining eligibility,
emphasizing the importance of determining optimal resulting in the exclusion of 49% of patients with chronic
dosing for clinical applications. In addition, preclinical HFrEF. The eligibility criteria for vericiguat set by the
9
safety data indicated that vericiguat was well-tolerated VICTORIA trial, guidelines, and regulatory labeling were
in animal models, showing no significant adverse effects designed to target a high-risk population with HFrEF in
at therapeutic doses. These promising results laid the a real-world setting. A consensus statement from India,
12
9
groundwork for human trials, including the VICTORIA based on expert opinions regarding the identification
Phase 3 trial, where vericiguat showed potential in and pharmacological management of worsening HF,
improving clinical outcomes for HF patients. In summary, concluded that vericiguat effectively reduces the risk of
9
preclinical studies highlight vericiguat’s mechanism of cardiovascular death and hospitalization for adults with
action, efficacy in restoring NO-sGC-cGMP signaling, symptomatic chronic HF and an ejection fraction of
dose-dependent effects, and favorable safety profile, <45%. This medication is recommended for individuals
13
strongly supporting its therapeutic role in chronic HF and who have been recently hospitalized for HF or require
possibly other cardiovascular conditions. 9 outpatient intravenous diuretics. The initial dose of
13
Volume 8 Issue 2 (2025) 48 doi: 10.36922/itps.3756