Page 31 - JCTR-10-1
P. 31
Shah et al. | Journal of Clinical and Translational Research 2024; 10(1): 25-32 27
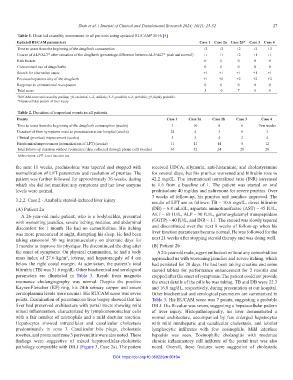
Table 1. Detailed causality assessment in all patients using updated RUCAM 2016 [5]
#
Updated RUCAM parameters Case 1 Case 2a Case 2b* Case 3 Case 4
Time to onset from the beginning of the drug/herb consumption +2 +2 +2 +2 +2
Course of ALP/ALT* after cessation of the drug/herb (percentage difference between ALP/ALT* peak and normal) +1 +1 +2 +1 +1
Risk factors 0 0 0 0 0
Concomitant use of drugs/herbs 0 0 0 0 0
Search for alternative cause +1 +1 +1 +1 +1
Previous hepatotoxicity of the drug/herb +1 +2 +2 +2 +2
Response to unintentional re-exposure 0 0 0 0 0
Total score 5 6 7 6 6
# RUCAM score and causality grading: ≤0, excluded; 1–2, unlikely; 3–5, possible; 6–8, probable; ≥9, highly probable.
*Hepatocellular pattern of liver injury
Table 2. Duration of important events in all patients
Events Case 1 Case 2a Case 2b Case 3 Case 4
Time to onset from the beginning of the drug/herb consumption (weeks) 3 10 8 6 Few weeks
Duration of from symptoms onset to presentation at our hospital (weeks) 24 4 3 6 3
Clinical (pruritus) improvement (weeks) 3 3 4 2 4
Biochemical improvement (normalization of LFT) (weeks) 11 11 14 8 12
Total follow-up duration without recurrence (data collected through phone call) (weeks) 36 32 24 28 28
Abbreviation: LFT: Liver function test.
the next 11 weeks, prednisolone was tapered and stopped with received UDCA, silymarin, anti-histaminic, and cholestyramine
normalization of LFT parameters and resolution of pruritus. The for several days, but his pruritus worsened and bilirubin rose to
patient was further followed for approximately 36 weeks, during 42.2 mg/dL. The international normalized ratio (INR) increased
which she did not manifest any symptoms and her liver enzyme to 1.6 from a baseline of 1. The patient was started on oral
levels were normal. prednisolone 40 mg/day and naltrexone for severe pruritus. Over
3 weeks of follow-up, his pruritus and jaundice improved. The
3.2.2. Case 2 - Anabolic steroid-induced liver injury results of LFT are as follows: TB – 10.6 mg/dL, direct bilirubin
(A) Patient 2a (DB) – 6.8 mL/dL, aspartate aminotransferase (AST) – 45 IU/L,
A 26-year-old male patient, who is a bodybuilder, presented ALT – 68 IU/L, ALP – 90 IU/L, gamma-glutamyl transpeptidase
with worsening jaundice, severe itching, malaise, and abdominal (GGTP) – 40 IU/L, and INR – 1.1. The steroid was slowly tapered
discomfort for 1 month. He had no comorbidities. His itching and discontinued over the next 8 weeks of follow-up when his
was more pronounced at night, disrupting his sleep. He had been liver function parameters became normal. He was followed for the
taking stanozolol 50 mg intramuscularly on alternate days for next 21 weeks after stopping steroid therapy and was doing well.
3 months to improve his physique. He discontinued the drug after (B) Patient 2b
the onset of symptoms. On physical examination, he had a body A 24-year-old male, a gym enthusiast without any comorbidities
mass index of 27.6 kg/m , icterus, and hepatomegaly of 4 cm approached us with worsening jaundice and severe itching, which
2
below the right costal margin. At admission, the patient’s total had persisted for 20 days. He had been taking creatine and some
bilirubin (TB) was 31.6 mg/dL. Other biochemical and serological steroid tablets for performance enhancement for 2 months and
parameters are illustrated in Table 3. Result from magnetic stopped after the onset of symptoms. The patient could not provide
resonance cholangiography was normal. Despite the positive the exact details of the pills he was taking. TB and DB were 22.3
Kayser-Fleischer (KF) ring, his 24-h urinary copper and serum and 16.8 mg/dL, respectively, during presentation at our hospital.
ceruloplasmin levels were normal. His RUCAM score was seven Other biochemical and serological parameters are summarized in
points. Examination of percutaneous liver biopsy showed that his Table 3. His RUCAM score was 7 points, suggesting a probable
liver had preserved architecture with portal tracts showing mild DILI. His R-value was seven, suggesting a hepatocellular pattern
mixed inflammation, characterized by lymphomononuclear cells of liver injury. Histopathologically, his liver demonstrated a
with a fair number of neutrophils and a mild ductular reaction. normal architecture, accompanied by few enlarged hepatocytes
Hepatocytes showed intracellular and canalicular cholestasis with mild intrahepatic and canalicular cholestasis, and lobular
predominantly in zone 3. Canalicular bile plugs, cholestatic lymphocytic infiltrates with few eosinophils. Mild interface
rosettes, and prominent zone 3 perivenulitis were also noted. These hepatitis was seen. Eosinophilic cholangitis with moderate
findings were suggestive of mixed hepatocellular-cholestatic chronic inflammatory cell infiltrate of the portal tract was also
pathology compatible with DILI (Figure 3, Case 2a). The patient noted. Overall, these features were suggestive of cholestatic
DOI: https://doi.org/10.36922/jctr.00104