Page 29 - JCTR-11-3
P. 29
Journal of Clinical and
Translational Research Immunogenicity and safety of flu vaccines
Figure 2. (Continued)
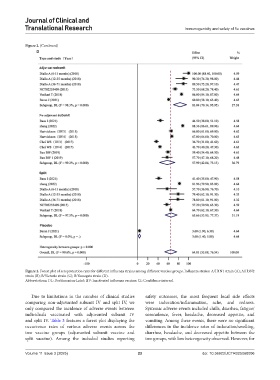
D
Figure 2. Forest plot of seroprotection rates for different influenza strains among different vaccine groups. Influenza strains: A/H1N1 strain (A); A/H3N2
strain (B); B/Victoria strain (C); B/Yamagata strain (D).
Abbreviations: DL: DerSimonian-Laird; IIV: Inactivated influenza vaccine; CI: Confidence interval.
Due to limitations in the number of clinical studies safety outcomes, the most frequent local side effects
comparing non-adjuvanted subunit IV and split IV, we were induration/inflammation, ache, and redness.
only compared the incidence of adverse events between Systemic adverse events included chills, diarrhea, fatigue/
individuals vaccinated with adjuvanted subunit IV somnolence, fever, headache, decreased appetite, and
and split IV. Table 3 features a forest plot displaying the vomiting. Among these events, there were no significant
occurrence rates of various adverse events across the differences in the incidence rates of induration/swelling,
two vaccine groups (adjuvanted subunit vaccine and diarrhea, headache, and decreased appetite between the
split vaccine). Among the included studies reporting two groups, with low heterogeneity observed. However, for
Volume 11 Issue 3 (2025) 23 doi: 10.36922/JCTR025060006